Langmuir Blodgett films as models of organized structures. Langmuir-Blodgett technology. Film production plants
Amphiphilic substances
Amphiphiles - chemical substances having both hydrophilic and hydrophobic parts. They are generally insoluble in water. The hydrophobic group is a large hydrocarbon fragment with a chain of the form CH 3 (CH 2) n (n>4). The hydrophilic group may consist of anionic carboxylates (RCO 2 -), sulfates (RSO 4 -), sulfonates (RSO 3 -) and cationic amines (RNH 3 +). There are also zwitterionic hydrophilic groups such as glycerol, DPPC phospholipids, etc. In addition, there are molecules that have several hydrophilic and hydrophobic groups, such as proteins and enzymes. Below is an example of typical amphiphiles at an air-water interface.
Langmuir monolayer
A Langmuir monolayer is a thick layer consisting of one molecule of insoluble organic material distributed throughout an aqueous subphase. Monomolecular layers are well studied and are used to form Langmuir Blodgett films (LB films), which are formed when a monolayer is deposited on a liquid phase.
Gibbs monolayer
The Gibbs monolayer is a partially soluble amphiphile. It differs from the Langmuir monolayer only in solubility. The substances that are used to form the Langmuir monolayer are insoluble, causing the molecules to settle at the air-water interface. In the Gibbs monolayer, the molecule "jumps" on the surface of the water. However, there is no rigid dividing line between these monolayers, since absolutely insoluble substances are very rare in nature. Separation of these two monolayers is only possible at water depth using an experimental scale.
Langmuir-Blodgett films
 Langmuir molecular film contains one or more monolayers of amphiphile deposited on the surface of a liquid by dipping a solid substrate into the liquid. Each new monolayer is deposited with each new dipping and pulling, which allows the formation of molecular films with a very precise thickness value. Monolayers usually consist of polar molecules - a hydrophilic head and a hydrophobic tail (example: fatty acids).
Langmuir molecular film contains one or more monolayers of amphiphile deposited on the surface of a liquid by dipping a solid substrate into the liquid. Each new monolayer is deposited with each new dipping and pulling, which allows the formation of molecular films with a very precise thickness value. Monolayers usually consist of polar molecules - a hydrophilic head and a hydrophobic tail (example: fatty acids).
This phenomenon was discovered in 1918 by Langmuir and Katherine Blodgett, after which, 16 years later, it was found that repetition of the experiment leads to layering.
The following are 3 types of Langmuir films that are produced by the vertical lift method.

In addition, there is also the Schaeffer horizontal lifting method. Here, the chute descends horizontally into the liquid, touches the monolayer, and moves horizontally to lift the film. In this case, the trough must be hydrophobic in nature.
Above is a diagram of the Schaeffer lifting method.

Surface pressure p is defined as p = S 0 - S f, where S 0 and S f - surface tension a clean air-water interface and a sub-phase with material distributed over it. It is actually a change in the surface tension of water due to the addition of another molecule at the air-water interface.
Isotherm Pressure (TT) - Area (A)
An isotherm consists of a curve of surface pressure and the area of a molecule at a fixed temperature. Bends and kinks indicate phase transitions.
In the isotherm figure, you can observe different areas that differ in compressibility. First, at low pressures, the molecules are in the gas phase (G). Then, with increasing pressure, a liquid appearance region (LE) appears. With an even greater increase in pressure, a section of liquid condensate appears. Further, with increasing pressure, a solid phase (S) is observed. Ultimately, the increase in pressure causes the monolayer to become unstable and collapse with a sharp decrease in pressure. For a particular molecule, each stage depends on its characteristic temperature and compression rate.

The transfer coefficient is defined as tr = Am/As, where Am- reduction of the monolayer during deposition, As is the surface area of the substrate. Ideally tr = 1.
Sustainability Diagram
The stability curve is the relative change in monolayer area over time at constant pressure. A stability curve can be obtained by measuring area (A) versus time (T) at constant pressure. The curve shows how stable the monolayer is, and also allows you to judge what processes occur in the monolayer at a certain point in time. The main characteristics of stability are also shown here.
Pressure vs. Time Graph (P - V - T)

This is a plot of pressure versus time, assuming that the monolayer area is constant and stable. The main function of the graph is to measure the adsorption kinetics of water molecules present in the subphase on pre-prepared surfaces of the monolayer. The figure below depicts the adsorption kinetics of a protein (ovalbumin) on various lipid monolayers (octadecylamine, stearic acid, DPPC).

Two Wilhelmy plates are used to measure surface pressure. One is made in the form of a paper filter, and the other is in the form of a plate with a rough surface. In our case, a filter paper plate is used, which is completely covered with water and has actually become a continuation of the subphase. In this case, it should be noted that the contact angle will be equal to zero. The platinum surface of the insert should be sandblasted. A rough platinum plate is completely wetted by water, so that the contact angle is zero. On a smooth surface, you will not get a zero contact angle. The plate must be very thin. The width of the plate is usually taken to be 1 cm.
Let a plate of length l, width w and thickness t be immersed in water for 1 hour. Next, the resulting force F comes into play, which acts on the plate.

where rho- plate density, rho 0- density of water, g- acceleration of gravity.
Now the surface pressure is determined p = S 0 - S f, where S0 And S f- surface tension of pure subphase and subphase with material.
The measurement of the force acting on a subphase is expressed as follows:
DF = 2 (w + t). DS = 2(w+t)p(given that h = const, qc ~ 0, which is why Cos qc =1)
If the plate is very thin, that is t negligible compared to w and if the slab width w=1cm, then DF = 2p or p = DF/2.
Thus, under these conditions, the surface pressure is half the weight measured on the microbalance after zeroing it in pure water.
Surface tension
Surface tension is a property of liquids, which is based on the adhesion force of asymmetric molecules on or near the surface, as a result of which the surface tends to compress and acquires the properties of a stretched elastic membrane.
The following are surface tension values in various systems at 293K (Weast, R. C. (Ed.). Handbook of Chemistry and Physics, 61st ed. Boca Raton, FL: CRC Press, p. F-45, 1981.).
Change in Surface Tension at an Air-Water Interface at a Certain Temperature (Weast, R. C. (Ed.). Handbook of Chemistry and Physics, 61st ed. Boca Raton, FL: CRC Press, p. F-45, 1981.).
| Temperature˚C | Surface tension (erg cm -2) |
| 0 | 75.6 |
| 5 | 74.9 |
| 10 | 74.22 |
| 15 | 73.49 |
| 18 | 73.05 |
| 20 | 72.75 |
| 25 | 71.97 |
| 30 | 71.18 |
| 40 | 69.56 |
| 50 | 67.91 |
| 60 | 66.18 |
| 70 | 64.4 |
| 80 | 62.6 |
| 100 | 58.9 |
Contact angle
The equilibrium contact angle of a liquid on a solid surface is measured at the line of contact of three phases (liquid, solid and gaseous).
For example, a film of water on glass has a zero contact angle, but if a film of water is on an oily or plastic surface, then the contact angle can be greater than 90°C.

Hydrophobic surfaces (figure A) are surfaces where the contact angle with water exceeds 90°C. If the contact angle with water is less than 90°C, then the surface is considered hydrophilic (figure B).
The foundations of modern ideas about monomolecular films were laid in the works of A. Pokels and Rayleigh in late XIX- the beginning of the 20th century.
Investigating the phenomena that occur on the water surface when it is contaminated with oil, Pockels found that the value of the surface tension of water depends on the area of the water surface and the volume of oil applied to the surface of the water.
Rayleigh, explaining the experimental results obtained by Pockels, suggested that when a sufficiently small volume of oil is applied to the water surface, it spontaneously spreads as a monomolecular layer, and when the water surface area decreases to the critical oil molecule, they form a densely packed structure touching each other, which leads to a decrease in values of the surface tension of water.
The greatest contribution to the study of monomolecular films was made by I. Langmuir. Langmuir was the first to systematically study floating monolayers on the surface of a liquid. Langmuir explained the results of experiments to reduce surface tension aqueous solutions in the presence of surfactants, in 1917. He developed the design of an instrument for direct measurement of internal pressure in a monolayer (Langmuir balance) and proposed a new experimental method for studying monomolecular layers. Langmuir showed that many water-insoluble amphiphilic substances, which are polar molecules organic matter containing a hydrophilic part - the “head” and a hydrophobic part - the “tail”, are capable of spreading over the water surface in a monomolecular layer to reduce its surface tension. Studying the dependence of surface pressure (surface pressure in a monolayer - the ratio of the intermolecular repulsion force of a film opposing compression to the unit length of the monolayer (N/m)) on the area of the monolayer, Langmuir discovered the existence of various phase states of the monolayer.
Monomolecular films of insoluble amphiphilic substances on the surface of a liquid are called Langmuir films.
In the early 1930s, C. Blodgett carried out the transfer of monomolecular films of insoluble fatty acids onto the surface of a solid substrate, thus obtaining multilayer films.
Blodgett's approach, based on the Langmuir technique, was called the Langmuir-Blodgett technology, and the films obtained in this way are called Langmuir-Blodgett films.
Consider a two-phase gas-liquid system.
The liquid molecules, being in the volume of the phase, experience the action of attractive forces (cohesion) from the surrounding molecules. These forces balance each other and their resultant is zero. Molecules located on the air-water interface experience the action of forces of different magnitudes from the side of the adjacent phases. The force of attraction per unit volume of liquid is much greater than the unit volume of air. Thus, the net force acting on a molecule on the surface of a liquid is directed inside the volume of the liquid phase, reducing the surface area to the minimum possible value under given conditions.
To increase the surface of a liquid, it is necessary to do some work to overcome the internal pressure of the liquid.
An increase in the surface is accompanied by an increase in the surface energy of the system, the Gibbs energy. An infinitesimal change in the Gibbs surface energy dG with an infinitesimal surface change dS at constant pressure p and temperature T is given by:
Where is the surface tension. So the surface tension
=(G/S)| T,p,n = const,
where n is the number of moles of components.
Energy definition: surface tension is the Gibbs specific free surface energy. Then the surface tension is equal to the work spent on the formation of a unit surface (J / m 2).
Force definition: surface tension is a force on the surface tangential to it and tending to reduce the surface of the body to the minimum possible for a given volume and conditions (N / m).
[J / m 2 \u003d N * m / m 2 \u003d N / m]
According to the second law of thermodynamics, the Gibbs energy of a system spontaneously tends to a minimum value.
As the temperature increases, the value of the surface tension of the gas-liquid interface decreases.
Let us consider the behavior of surface tension at the gas-liquid interface in the presence of a surfactant.
Substances whose presence at the phase boundary leads to a decrease in the value of surface tension are called surfactants.
Surfactants have an asymmetric molecular structure, which consists of polar and non-polar groups. The polar group has a dipole moment and has an affinity for the polar phase. The groups -COOH, -OH, -NH 2, -CHO, etc. have polar properties.
The non-polar part of the surfactant molecule is a hydrophobic hydrocarbon chain (radical).
Surfactant molecules spontaneously form an oriented monolayer on the phase interface in accordance with the condition for reducing the Gibbs energy of the system: polar groups are located in the aqueous (polar) phase, and hydrophobic radicals are displaced from the aqueous medium and pass into a less polar phase - air.
Surfactant molecules, especially their hydrocarbon radicals, being at the air-water interface, interact weakly with water molecules than water molecules with each other. Thus, the total contracting force per unit length decreases, resulting in a decrease in the value of surface tension compared to a pure liquid.
The setup for studying Langmuir films and obtaining Langmuir-Blodgett films includes the following main units:
a container that contains a liquid (subphase), called a bath,
surface barriers moving in opposite directions along the edges of the bath,
Wilhelmy electronic scales, for measuring the surface pressure in a monolayer,
substrate moving device.
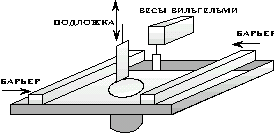
The bath itself is usually made of polytetrafluoroethylene (PTFE), which provides chemical inertness and prevents the possibility of subphase leakage. The material for the manufacture of barriers can also be a hydrophobic fluoroplastic, or another chemically inert material.
Thermal stabilization is carried out by water circulation through a system of channels located under the bottom of the bath.
The unit is located on a vibration-protective base in a specialized room with an artificial climate - a “clean room”. All chemicals used must be of the highest purity.
To measure the surface pressure in a monolayer in modern Langmuir-Blodgett installations, a surface pressure sensor is used - Wilhelmy electronic balance.
The operation of the sensor is based on the principle of measuring the force necessary to compensate for the impact on the Wilhelmy plate of the surface pressure force in the monolayer at the “subphase-gas” interface.
Consider the forces acting on the Wilhelmy plate.

W, l, t are the width, length, and thickness of the Wilhelmy plate, respectively; h is the depth of immersion in water.
The resulting force acting on the Wilhelmy plate consists of three components: Force = weight - Archimedes force + surface tension.
F=glwt-’ghwt+2(t+w)cos ,
where ,’ is the density of the plate and subphase, respectively, is the contact wetting angle, g is the acceleration free fall. The material of the Wilhelmy plate is chosen so that =0.
Surface pressure is the difference between the force acting on a plate immersed in pure water and the force acting on a plate immersed in water, the surface of which is covered with a monolayer:
where ' is the surface tension of pure water. The Wilhelmy plate is characterized by t< F/2t=mg/2t [N/m], where m is the value measured by the Wilhelmy balance. A feature of the Langmuir-Blodgett method is that a continuous ordered monomolecular layer is preliminarily formed on the subphase surface and subsequently transferred to the substrate surface. The formation of an ordered monolayer on the subphase surface proceeds as follows. A certain volume of a solution of the test substance in a highly volatile solvent is applied to the surface of the subphase. After evaporation of the solvent, a monomolecular film is formed on the water surface, the molecules in which are arranged randomly. At a constant temperature T, the state of the monolayer is described by the compression isotherm -A, which reflects the relationship between the surface pressure of the barrier and the specific molecular area A. With the help of a movable barrier, the monolayer is compressed to obtain a continuous film with dense packing of molecules, in which the specific molecular area A is approximately equal to the cross-sectional area of the molecule, and hydrocarbon radicals are oriented almost vertically. The linear sections on the dependence -A, corresponding to the compression of the monolayer in various phase states, are characterized by the value A 0 - the area per molecule in the monolayer, obtained by extrapolating the linear section to the A axis (=0 mN/m). It should be noted that the phase state of a monolayer of amphiphilic substance (AMPS) localized at the “subphase-gas” interface is determined by the adhesive-cohesive balance of forces in the “subphase-monolayer” system and depends on the nature of the substance and the structure of its molecules, temperature T, and subphase composition. Gaseous G, liquid L1, liquid-crystalline L2 and solid-crystalline S monolayers are isolated. The formed monolayer, consisting of close-packed AMPB molecules, is transferred to a solid substrate moving up and down through the water surface. Depending on the type of substrate surface (hydrophilic or hydrophobic) and the sequence in which the substrate intersects the subphase surface with and without a monolayer, one can obtain PLBs with a symmetric (Y) or asymmetric (X, Z) structure. The value of the surface pressure , at which the monolayer is transferred to the substrate, is determined from the compression isotherm of the given AMPI and corresponds to the state with close packing of molecules in the monolayer. During the transfer, the pressure is kept constant by reducing the area of the monolayer by moving barriers. The criterion for the degree of coverage of the substrate with a monolayer is the transfer coefficient k, which is determined by the formula: where S', S" are the area of the monolayer at the moment of the beginning of the transfer and after the end of the transfer, respectively, Sn is the area of the substrate. To obtain a Langmuir-Blodgett film uniform in thickness, the substrate surface must have a roughness Rz<=50нм. Introduction
Langmuir-Blodgett films are a fundamentally new object of modern physics, and any of their properties are unusual. Even simple films composed of identical monolayers have a number of unique features, not to mention specially constructed molecular ensembles. Langmuir-Blodgett films find various practical applications in various fields of science and technology: electronics, optics, applied chemistry, micromechanics, biology, medicine, etc. Langmuir monolayers are successfully used as model objects for studying the physical properties of ordered two-dimensional structures. The Langmuir-Blodgett method makes it quite easy to change the surface properties of a monolayer and form high-quality film coatings. All this is possible due to precise control of the thickness of the resulting film, uniformity of the coating, low roughness and high adhesion of the film to the surface if the right conditions are selected. The properties of the films can also be easily varied by changing the structure of the polar head of the amphiphilic molecule, the composition of the monolayer, as well as the isolation conditions - the composition of the subphase and surface pressure. The Langmuir-Blodgett method makes it possible to incorporate various molecules and molecular complexes, including biologically active ones, into a monolayer. 1. This story begins with one of the many hobbies of Benjamin Franklin, an eminent American scientist and respected diplomat. While in Europe in 1774, where he settled another conflict between England and the North American States, Franklin experimented in his spare time with oil films on the surface of the water. The scientist was pretty surprised when it turned out that just one spoon of oil spreads over the surface of a half-acre pond (1 acre ≈ 4000 m 2). If we calculate the thickness of the formed film, it turns out that it does not exceed ten nanometers (1 nm = 10 -7 cm); in other words, the film contains only one layer of molecules. This fact, however, was realized only 100 years later. A certain inquisitive Englishwoman named Agnes Pockels in her own bathtub began to measure the surface tension of water contaminated with organic impurities, and simply speaking, with soap. It turned out that a continuous soap film noticeably lowers the surface tension (recall that it represents the energy of the surface layer per unit area). Pockels wrote about her experiments to the famous English physicist and mathematician Lord Rayleigh, who sent a letter to a reputable journal, providing his comments. Then Rayleigh himself reproduced the experiments of Pockels and came to the following conclusion: "The observed phenomena are beyond the scope of the Laplacian theory, and their explanation requires a molecular approach." In other words, relatively simple - phenomenological - considerations turned out to be insufficient, it was necessary to involve ideas about the molecular structure of matter, which were then far from obvious and not generally accepted. Soon the American scientist and engineer Irving Langmuir (1881-1957) appeared on the scientific scene. His entire scientific biography refutes the well-known “definition”, according to which “a physicist is someone who understands everything, but knows nothing; the chemist, on the contrary, knows everything and understands nothing, while the physicochemist neither knows nor understands. Langmuir was awarded the Nobel Prize precisely for his work on physical chemistry, remarkable for its simplicity and thoughtfulness. In addition to the classic results obtained by Langmuir in the field of thermionic emission, vacuum technology and absorption, he developed many new experimental techniques that confirmed the monomolecular nature of surface films and even made it possible to determine the orientation of molecules and the specific area occupied by them. Moreover, Langmuir was the first to start transferring films one molecule thick - monolayers - from the surface of water onto solid substrates. Subsequently, his student Katharina Blodgett developed a technique for repeatedly transferring one monolayer after another, so that a stacked stack structure, or multilayer, was obtained on a solid substrate, now called the Langmuir-Blodgett film. The name “Langmuir film” is often retained behind a monolayer lying on the water surface, although it is also used in relation to multilayer films. 2 Mermaid Molecules
It turns out that sufficiently complex molecules have their own addictions. For example, some organic molecules "like" contact with water, while others avoid such contact, being "afraid" of water. They are called respectively - hydrophilic and hydrophobic molecules. However, there are also molecules like mermaids - one part of them is hydrophilic, and the other is hydrophobic. Mermaid molecules must decide for themselves the problem: to be in water or not to be (if we are trying to prepare their aqueous solution). The solution found turns out to be truly Solomonic: of course, they will be in the water, but only half. Mermaid molecules are located on the surface of the water so that their hydrophilic head (which, as a rule, has separated charges - an electric dipole moment) is lowered into the water, and the hydrophobic tail (usually a hydrocarbon chain) protrudes out into the surrounding gaseous medium (Fig. 1) . The position of the mermaids is somewhat inconvenient, but it satisfies one of the basic principles of the physics of systems of many particles - the principle of minimum free energy and does not contradict our experience. When a monomolecular layer is formed on the water surface, the hydrophilic heads of the molecules are lowered into the water, and the hydrophobic tails stick out vertically above the water surface. One should not think that only some exotic substances have a tendency to be located in two phases at once (aqueous and non-aqueous), the so-called amphiphilicity. On the contrary, chemical synthesis methods can, at least in principle, "sew" a hydrophobic tail to almost any organic molecule, so that the range of mermaid molecules is extremely wide, and all of them can have a wide variety of purposes. 3. There are two ways of transferring monolayers to solid substrates, both of which are suspiciously simple as they can be done literally with bare hands. Monolayers of amphiphilic molecules can be transferred from the water surface to a solid substrate by the Langmuir-Blodgett method (top) or the Schaeffer method (bottom). The first method consists in "piercing" the monolayer with a vertically moving substrate. It makes it possible to obtain layers of both X - (molecular tails are directed towards the substrate) and Z-type (reverse direction). The second way is simply to touch the monolayer with a horizontally oriented substrate. It gives X-type monolayers. The first method was invented by Langmuir and Blodgett. The monolayer is turned into a liquid crystal with the help of a floating barrier - it is brought into a two-dimensional liquid crystal state, and then it is literally pierced with a substrate. In this case, the surface to which the film is to be transferred is oriented vertically. The orientation of the mermaid molecules on the substrate depends on whether the substrate is lowered through the monolayer into water or, conversely, lifted from water into air. If the substrate is immersed in water, then the tails of the “mermaids” turn out to be directed towards the substrate (Blodgett called such a construction an X-type monolayer), and if they are pulled out, then, on the contrary, away from the substrate (Z-type monolayer), Fig. 2a. By repeating the transfer of one monolayer after another under different conditions, it is possible to obtain multilayer stacks of three different types (X, Y, Z), which differ from each other in their symmetry. For example, in X- and Z-type multilayers (Fig. 3) there is no reflection-inversion center, and they have a polar axis directed away from the substrate or towards the substrate, depending on the orientation of the positive and negative electric charges separated in space, that is, in depending on the direction of the electric dipole moment of the molecule. Multilayers of the Y-type are composed of double layers, or, as they say, bilayers (by the way, they are built similarly to biological membranes), and turn out to be centrally symmetrical. Multilayer structures of X-, Z-, and Y-types differ in the orientation of molecules relative to the substrate. Structures of X- and Z-types are polar, since all molecules "look" in the same direction (the tails - to the substrate or away from the substrate for X- and Z-types, respectively). Rice. 3. X- and Z-type structures the structure corresponds to a non-polar two-layer package resembling the structure of a biological membrane. The second method was proposed by Schaeffer, also a student of Langmuir. The substrate is oriented almost horizontally and is brought into light contact with the monolayer, which is retained in the solid phase (Fig. 2b). The monolayer simply adheres to the substrate. By repeating this operation, an X-type multilayer can be obtained. On Fig. Figure 4 shows the process of monolayer deposition when the substrate is lifted from the subphase: the hydrophilic heads of the amphiphilic molecules "stick" to the substrate. If the substrate is lowered from the air into the subphase, then the molecules "stick" to it with hydrocarbon tails. . Film production plants General block diagram of the Langmuir installation 1 - Langmuir bath; 2 - transparent sealed box; Massive metal base plate; 4 - shock absorbers; Movable barrier; 6 - scales Wilhelmy; 7 - plate weights Wilhelmy; 8 - substrate; 9 - electric drive of the barrier (5); - electric drive of the substrate (8); II - peristaltic pump; - ADC / DAC interface with power amplifiers; Personal computer IBM PC/486.
The installation is controlled through a personal computer using a special program. To measure the surface pressure, Wilhelmy balances are used (the surface pressure of a monolayer p is the difference between the surface tensions on a clean water surface and on a surface covered with a surfactant monolayer). In fact, the Wilhelmy balance measures the force F=F 1 +F 2 with which a plate wetted in water is drawn into the water (see Fig. 7). A piece of filter paper is used as a wettable plate. The voltage at the output of the Wilhelmy balance is linearly related to the surface pressure p. This voltage is supplied to the input of the ADC installed in the computer. The monolayer area is measured using a rheostat, the voltage drop across which is directly proportional to the coordinate value of the movable barrier. The signal from the rheostat is also fed to the input of the ADC. To carry out sequential transfer of a monolayer from the water surface to a solid substrate with the formation of multilayer structures, a mechanical device (10) is used that slowly (at a speed of several mm per minute) lowers and raises the substrate (8) through the surface of the monolayer. As the monolayers are sequentially transferred to the substrate, the amount of the monolayer-forming substance on the water surface decreases, and the movable barrier (5) moves automatically, maintaining the surface pressure constant. The movable barrier (5) is controlled via a computer using the voltage supplied from the DAC output through a power amplifier to the corresponding motor. The movement of the substrate is controlled from the control panel using the knobs for coarse and smooth adjustment of the substrate speed. The supply voltage is supplied from the power supply to the control panel, and from there through the power amplifier to the electric motor of the lifting mechanism. Automated installation KSV 2000 The technique for obtaining Langmuir-Blodgett films includes many elementary technological operations, i.e. elementary influences on the system from the outside, as a result of which structure-forming processes take place in the “subphase - monolayer - gas - substrate” system, which ultimately determine the quality and properties of multistructures. To obtain films, an automated KSV 2000 installation was used. The scheme of the installation is shown in Fig. 8. Rice. 8. Installation diagram KSV 2000 Under the protective cap 1 there is a symmetrical three-section Teflon cell 2 on the anti-vibration table 11, on the sides of which the counter-coordinated movement of Teflon barriers 5 is carried out. barriers 8 and ensures the maintenance of a given surface pressure (determined from the compression isotherm and corresponding to the ordered state of the monolayer) in the process of monolayer transfer to the substrate surface. The substrate 3 is clamped in the holder at a certain angle to the surface of the subphase and is moved by the device 10 (equipped with a mechanism for transferring the substrate between the sections of the cuvette) using the drive 9. Before the technological cycle, the surface of the subphase 12 is preliminarily prepared by cleaning with the help of a pump 13. The installation is automated and equipped with a computer 14. The main part of the installation - a Teflon cell (a top view is shown in Fig. 9) - consists of three compartments: two of the same size for spraying various substances into the subphase and one small one with a clean surface. The presence of a three-section cuvette in the presented setup, a mechanism for transferring the substrate between sections, and two independent barrier control channels makes it possible to obtain mixed Langmuir films consisting of monolayers of various substances. On Fig. 10 shows one of two identical cell compartments with a surface pressure sensor and barriers. The surface area of the monolayer changes due to the movement of the barriers. The barriers are made of Teflon and are heavy enough to prevent leakage of the monolayer under the barrier.
Rice. 10. Cuvette compartment Technical characteristics of the installation: Maximum substrate size 100*100 mm Film deposition speed 0.1-85mm/min Number of deposition cycles 1 or more Film drying time in cycle 0-10 4 sec Surface measuring area 0-250 mN/m pressure Measurement accuracy 5 µN/m surface pressure Large installation bay area 775*120mm Subphase volume 5.51 l Temperature control of subphase 0-60 °C Barrier speed 0.01-800 mm/min 5. Factors affecting the quality of Langmuir-Blodgett films
The quality factor of Langmuir-Blodgett films is expressed as follows way: K \u003d f (K us, K those, K pav, K ms, Kp), mc - measuring devices; Kteh - technological purity; Kpaw is the physicochemical nature of the surfactant sprayed onto the subphase; K ms is the phase state of the monolayer on the surface of the subphase; Kp - type of substrate. The first two factors are related to design and technological, and the rest - to physical and chemical. Measuring devices include devices for moving the substrate and the barrier. The requirements for them when forming multistructures are as follows: No mechanical vibrations; The constancy of the speed of movement of the sample; The constancy of the speed of movement of the barrier; Maintaining a high level of technological purity Control of the purity of raw materials (use of distilled water as the basis of the subphase, preparation of solutions of surfactants and electrolytes immediately before their use); Carrying out preparatory operations, such as etching and cleaning of substrates; Preliminary cleaning of the surface of the subphase; Creation of a quasi-closed volume in the working area of the installation; Carrying out all work in a specialized room with an artificial climate - a "clean room". The factor that determines the physicochemical nature of a surfactant characterizes such individual properties of the substance as: The structure (geometry) of a molecule, which determines the ratio of hydrophilic and hydrophobic interactions between the molecules of the surfactant itself and the molecules of the surfactant and subphase; Solubility of surfactants in water; Chemical properties of surfactants To obtain films of high structural perfection, it is necessary to control the following parameters: surface tension in the monolayer and transfer coefficient characterizing the presence of defects in the PLB; temperature, pressure and humidity of the environment, PH subphases, Film deposition rate The compressibility factor for isotherm sections, defined as follows: where (S, P) are the coordinates of the beginning and end of the linear section of the isotherm. 6. Unique film properties
A multilayer is a fundamentally new object of modern physics, and therefore any of their properties (optical, electrical, acoustic, etc.) are completely unusual. Even the simplest structures composed of identical monolayers have a number of unique features, not to mention specially constructed molecular ensembles. As soon as we already know how to obtain a monolayer of identically oriented molecules on a solid substrate, there is a temptation to connect an electric voltage source or, say, a measuring device to it. Then we actually connect these devices directly to the ends of the individual molecule. Until quite recently, such an experiment was impossible. An electric field can be applied to the monolayer and the shift of the optical absorption bands of the substance can be observed or the tunneling current in the external circuit can be measured. Connecting a voltage source to the monolayer through a pair of film electrodes leads to two very pronounced effects (Fig. 11). First, the electric field changes the position of the light absorption bands of the molecule on the wavelength scale. This is the classic Stark effect (named after the famous German physicist who discovered it in 1913), which, however, in this case has interesting features. The point is that the direction of the shift of the absorption band depends, as it turned out, on the mutual orientation of the electric field vector and the intrinsic dipole moment of the molecule. And this is what this leads to: for the same substance and, moreover, for the same field direction, the absorption band shifts to the red region for an X-type monolayer and to blue for a Z-type monolayer. Thus, the orientation of the dipoles in the monolayer can be judged from the direction of the band shift. Qualitatively, this physical situation is understandable, but if we try to interpret the shifts of the bands quantitatively, the most interesting question arises of how exactly the electric field is distributed along a complex molecule. The theory of the Stark effect is built on the assumption of point atoms and molecules (this is natural - after all, their sizes are much smaller than the length over which the field changes), but here the approach should be radically different, and has not yet been developed. Another effect consists in the passage of a tunneling current through a monolayer (we are talking about the mechanism of quantum mechanical leakage of electrons through a potential barrier). At low temperatures, the tunneling current through the Langmuir monolayer is indeed observed. The quantitative interpretation of this purely quantum phenomenon must also include the complex configuration of the mermaid molecule. And what can the connection of a voltmeter to a monolayer give? It turns out that then it is possible to monitor the change in the electrical characteristics of the molecule under the influence of external factors. For example, illumination of a monolayer is sometimes accompanied by a noticeable charge redistribution in each molecule that has absorbed a light quantum. This is the effect of the so-called intramolecular charge transfer. A quantum of light, as it were, moves an electron along a molecule, and this induces an electric current in the external circuit. The voltmeter thus registers the intramolecular electronic photoprocess. Intramolecular movement of charges can also be caused by changing the temperature. In this case, the total electric dipole moment of the monolayer changes, and the so-called pyroelectric current is recorded in the external circuit. We emphasize that none of the described phenomena is observed in films with a random distribution of molecules over orientations. Langmuir films can be used to simulate the effect of light energy concentration on a selected molecule. For example, at the initial stage of photosynthesis in green plants, light is absorbed by certain types of chlorophyll molecules. Excited molecules live long enough, and self-excitation can move through the same type of densely spaced molecules. This excitation is called an exciton. The "walk" of the exciton ends at the moment it enters the "wolf pit", the role of which is played by a chlorophyll molecule of a different type with a slightly lower excitation energy. It is to this chosen molecule that energy is transferred from many excitons excited by light. The light energy collected from a large area is concentrated on a microscopic area - a "funnel for photons" is obtained. This funnel can be modeled using a monolayer of light-absorbing molecules interspersed with a small number of exciton interceptor molecules. After capturing an exciton, the interceptor molecule emits light with its characteristic spectrum. Such a monolayer is shown in Fig. 12a. When it is illuminated, one can observe the luminescence of both molecules - light absorbers, and molecules - interceptors of excitons. The intensity of the luminescence bands of molecules of both types is approximately the same (Fig. 12b), although their numbers differ by 2–3 orders of magnitude. This proves that there is a mechanism of energy concentration, that is, the effect of a photon funnel. Today, the scientific literature is actively discussing the question: is it possible to make two-dimensional magnets? And in physical language, we are talking about whether there is a fundamental possibility that the interaction of molecular magnetic moments located in the same plane will cause spontaneous magnetization. To solve this problem, transition metal atoms (for example, manganese) are introduced into amphiphilic mermaid molecules, and then monolayers are obtained by the Blodgett method and their magnetic properties are studied at low temperatures. The first results indicate the possibility of ferromagnetic ordering in two-dimensional systems. And one more example demonstrating the unusual physical properties of Langmuir films. It turns out that at the molecular level it is possible to carry out the transfer of information from one monolayer to another, neighboring one. After that, the adjacent monolayer can be separated and thus get a copy of what was "recorded" in the first monolayer. This is done in the following way. Let, for example, we obtained by the Blodgett method a monolayer of such molecules that are capable of pairing - dimerizing - under the influence of external factors, for example, an electron beam (Fig. 13). Unpaired molecules will be considered zeros, and paired ones - units of the binary information code. Using these zeros and ones, one can, for example, write an optically readable text, since unpaired and paired molecules have different absorption bands. Now we will apply the second monolayer to this monolayer using the Blodgett method. Then, due to the peculiarities of intermolecular interaction, molecular pairs attract exactly the same pairs to themselves, and single molecules prefer single ones. As a result of the work of this "interest club", the information picture will be repeated on the second monolayer. By separating the top monolayer from the bottom, you can get a copy. Such a copying process is quite similar to the process of replicating information from DNA molecules - the keepers of the genetic code - to RNA molecules that transfer information to the site of protein synthesis in the cells of living organisms. Conclusion
Why hasn't the LB method been implemented everywhere yet? Because there are pitfalls along the seemingly obvious path. The LB technique is outwardly simple and cheap (no ultrahigh vacuum, high temperatures, etc. are needed), but initially it requires significant costs to create especially clean rooms, since any dust grain that has settled even on one of the monolayers in the heterostructure is an incurable defect . The structure of a monolayer of a polymeric material, as it turned out, significantly depends on the type of solvent in which the solution is prepared for application to the bath. There is now an understanding of the principles according to which it is possible to plan and carry out the design and manufacture of nanostructures using Langmuir technology. However, new methods for studying the characteristics of already fabricated nanodevices are required. Therefore, we will be able to make greater progress in the design, manufacture and assembly of nanostructures only after we better understand the patterns that determine the physicochemical properties of such materials and their structural conditionality. Traditionally, X-ray and neutron reflectometry and electron diffraction are used to study LB films. However, the diffraction data is always averaged over the area on which the radiation beam is focused. Therefore, they are currently supplemented by atomic force and electron microscopy. Finally, the most recent advances in structural research are related to the launch of synchrotron sources. Stations began to be created in which an LB bath and an X-ray diffractometer are combined, due to which the structure of monolayers can be studied directly in the process of formation on the water surface. Nanoscience and the development of nanotechnologies are still at the initial stage of development, but their potential prospects are wide, research methods are constantly being improved, and there is no end to the work ahead. Literature monolayer film Langmuir Blodgett 1. Blinov L.M. "Physical properties and applications of Langmuir mono- and multi-molecular structures". advances in chemistry. v. 52, no. 8, p. 1263…1300, 1983. 2. Blinov L.M. "Langmuir films" Uspekhi Fizicheskikh Nauk, vol. 155, no. 3 p. 443…480, 1988. 3. Savon I.E. Diploma work // Study of the properties of Langmuir films and their production. Moscow 2010 pp. 6-14 -- [ Page 1 ] -- As a manuscript Specialty: 01.04.18 - crystallography, crystal physics Dissertations for the degree of Doctor of Physical and Mathematical Sciences Moscow 2012 www.sp-department.ru The work was carried out at the Federal State Budgetary Institution of Higher Professional Education "Ivanovo State University". Official Opponents: Ostrovsky Boris Isaakovich, Doctor of Physical and Mathematical Sciences, Federal State Budgetary Institution of Science Institute of Crystallography named after A.I. A.V. Shubnikov of the Russian Academy of Sciences, Leading Researcher of the Laboratory of Liquid Crystals Dadivanyan Artyom Konstantinovich, Doctor of Physical and Mathematical Sciences, Professor, Federal State Budgetary Institution of Higher Professional Education "Moscow State Regional University", Professor of the Department of Theoretical Physics Chvalun Sergey Nikolaevich, Doctor of Chemical Sciences, State Scientific Center of the Russian Federation “Scientific Research Institute of Physics and Chemistry named after A.I. L.Ya. Karpov, Head of the Laboratory of Polymer Structure Lead organization: Federal State Unitary Enterprise "Research Institute for Physical Problems named after V.I. F.V. Lukina, Zelenograd The defense will take place in 2012 at h. min. at a meeting of the dissertation council D 002.114.01 at the Federal State Budgetary Institution of Science Institute of Crystallography. A.V. Shubnikov of the Russian Academy of Sciences at the address 119333 Moscow, Leninsky pr., 59, conference hall The dissertation can be found in the library of the Federal State Budgetary Institution of Science of the Institute of Crystallography. A.V. Shubnikov of the Russian Academy of Sciences. Scientific Secretary of the Dissertation Council Candidate of Physical and Mathematical Sciences V.M. Kanevsky www.sp-department.ru GENERAL DESCRIPTION OF WORK It is important to study the features of their behavior under various conditions, the possibility of stabilization while maintaining lability within certain limits, etc. The study of the structure is a necessary link in the study of any materials, since their properties can be determined at various structural levels in the hierarchy: molecular, supramolecular, macroscopic. When solving structural problems, diffraction methods and, in particular, X-ray diffraction analysis are the most informative. However, due to the specifics of X-ray diffraction spectra of LCs (a small number of reflections, some of which, and in some cases all of them, can be diffuse), direct methods for determining the structure developed for crystalline objects are ineffective. In such a situation, a model approach to interpreting the diffraction spectra of both bulk liquid crystal objects and films based on mesogenic molecules seems to be more promising, and the development of new methods and approaches to solving structural problems for such systems is an important and urgent problem. Goals and tasks work. The aims of this work are to establish a correlation in the structures of bulk samples and LB films based on mesogenic molecules of various nature and to study the possibilities of obtaining stable quasi-two-dimensional functionally active film systems with a given architecture using LB technology. The achievement of the designated goals is realized through the solution of tasks related to: 1) with methods for orienting LC objects (including polymeric LCs) in the bulk and film state for structural studies and with the implementation of these methods at the device level; 2) with consideration of the structure of liquid crystal phases in terms of statistical models that take into account translational disturbances in the structure, and with structural modeling of layered systems for studying liquid crystal phases and LB films; 3) with stabilization of artificially formed film quasi-two-dimensional systems; 4) with prediction of the polar properties of chiral LC and LB films based on diffraction data; 5) with the formation of stable multilayer structures with isolated transport channels based on mesogenic ionophore molecules; 6) with the study of the temperature behavior of magnetically and electrically oriented mesogenic complexes of lanthanides; 7) with consideration of the formation of floating layers based on metal complexes in the presence of a magnetic field, including in "guest-host" systems, and their use to create macroscopically biaxial LB films. Scientific novelty 1. A model approach has been developed for determining the layer structure of smectics and LB films from small-angle scattering data, based on software modeling of a structure-forming fragment and using the obtained arrays of atomic coordinates to calculate interlayer diffraction, followed by fitting the structural model through changing the basic parameters (tilt, azimuthal angle, overlap in layers, conformation). 2. Parallel studies of bulk samples, floating layers, and LB films based on mesogens of various types made it possible to establish correlation relationships for bulk and film structures and show the dependence of the structure of the formed multilayer film on conformational transformations in the monolayer during its transfer to the substrate. 3. The possibility of obtaining stable LB films with a polar structure and corresponding properties from UV polymerized monolayers of mesogenic chiral and achiral acrylates and their mixtures and the advantage of this method over UV polymerization of multilayer LB films based on acrylates is shown; at which the mechanism of UV polymerization may not start due to the screening of C = C bonds when the end fragments of molecules in adjacent layers overlap. 4. It has been shown that the introduction of hydrogen bonding-active groups into the structure of para-substituted crown ethers significantly affects the structure of the crystalline phase and can be used to stabilize the quasi-two-dimensional film structure of LB films. 5. It has been shown that LB films of mesogenic crown ethers obtained on subphases of salts of unsaturated acids have a quasi-two-dimensional structure with salt molecules incorporated into the layers in a regular manner. 6. A two-phase behavior of a liquid-crystal dysprosium complex stimulated by a magnetic field has been discovered. 7. The orienting effect of the magnetic field in Langmuir monolayers of mesogenic lanthanide complexes was discovered, and on their basis LB films with a biaxial texture were obtained, including those in the guest-host system. Practical significance
1. The developed diffraction techniques can be used to study the structure of new liquid crystal compounds and thin multilayer films formed on their basis. 2. The results on the stabilization of quasi-two-dimensional film structures can be used, for example, in the design of nanoscale film functional elements. 3. The results of structural studies of chiral liquid crystal compounds in bulk samples and LB films can be useful in the development of new ferroelectric film materials. 5. The discovered two-phase behavior of lanthanide complexes oriented by a magnetic field in the liquid-crystal state provides additional possibilities for controlling the structure of these compounds and can be used in the development, for example, of magnetic gates. 6. It is shown that using lanthanide complexes as magnetically controlled elements in a floating layer, it is possible to obtain biaxial LB films, including films with nanosized conducting channels with a given azimuthal orientation in the layer. Provisions for defense Methodical approaches in diffraction studies of bulk and film LC systems based on statistical description and computer simulation of their structure. Results of studies of the structure (structural models) of bulk phases and LB films of monomeric and polymeric systems based on mesogens of various nature. Methodical approaches for obtaining (including stabilization) stable quasi-two-dimensional film structures. Results of predicting the ferroelectric behavior of a quasi-two-dimensional film structure based on the analysis of small-angle X-ray scattering data and structural modeling. Results of structural studies of LB films based on mesogenic crown ethers and their complexes with salts of fatty acids. Results of studies of structural-phase transformations in LC phases of oriented lanthanide complexes and LB films based on them. Methodical approaches and results on obtaining biaxial LB films. Approbation of work The results of the work were presented at the IV (Tbilisi, 1981) and V (Odessa, 1983) International Conferences of Socialist Countries on Liquid Crystals; IV, V (Ivanovo, 1977, 1985) and VI (Chernigov, 1988) All-Union conferences on liquid crystals and their practical use; European Summer Conference on Liquid Crystals (Vilnius, Lithuania, 1991); III All-Russian Symposium on Liquid Crystalline Polymers (Chernogolovka, 1995); 7th (Italy, Ancona, 1995) and 8th (Asilomar, California, USA, 1997) International Conferences on Organized Molecular Films; II International Symposium "Molecular Order and Mobility in Polymer Systems" (St. Petersburg, 1996), 15th (Budapest, Hungary, 1994), 16th (Kent, Ohio, USA, 1996), 17th (Strasbourg, France, 1998) and the 18th (Sindai, Japan, 2000) International Conferences on Liquid Crystals; 3rd European Conference on Molecular Electronics (Leuven, Belgium, 1996); European Winter Conference on Liquid Crystals (Poland, Zakopane, 1997); I International Scientific and Technical Conference “Ecology of Man and Nature” (Ivanovo, 1997); 6th (Brest, France, 1997) and 7th (Darmstadt, Germany, 1999) International Conferences on Ferroelectric Liquid Crystals; IX International Symposium "Thin Films in Electrical Engineering" (Plyos, Russia, 1998); I All-Russian Conference "Surface Chemistry and Nanotechnology" (St. Petersburg - Khilovo, 1999); III All-Russian scientific conference "Molecular physics of non-equilibrium systems" (Ivanovo, 2001); II International Symposium "Molecular Design and Synthesis of Supramolecular Architectures" (Kazan, Russia, 2002); Spring Conferences of the European Society for Research in Materials (Strasbourg, France, 2004 and 2005); VI, VII and VIII National conferences on the use of X-ray, synchrotron radiation, neutrons and electrons for the study of materials (Moscow, Russia 2007, 2009, 2011); V International scientific conference “Kinetics and mechanism of crystallization. Crystallization for Nanotechnologies, Engineering and Medicine” (Ivanovo, Russia 2008); III, IV, V and VII International conferences on lyotropic liquid crystals (Ivanovo, Russia, 1997, 2000, 2003 and 2009). Personal contribution Applicant The Applicant plays the main role in choosing the areas that are the subject of the submitted work, setting tasks and developing methodological approaches for their solution, setting up experiments (including design work) and calculations. The main results of experimental studies included in the work were obtained by the applicant personally or with his direct participation, which was reflected in joint publications with T.V. Pashkova and his graduate students V.M. Dronov, A.V. Kurnosov, A.V. Krasnov, A.V. Pyatunin and in the Ph.D. theses defended by them. Publications 41 papers were published on the topic of the dissertation (including 15 in peer-reviewed foreign journals and 19 papers in scientific journals on the list of the Higher Attestation Commission), an author's certificate for the invention was obtained (the list of publications is given at the end of the abstract). Structure and scope of work The dissertation consists of an introduction, six chapters and a list of cited literature. The total volume of the dissertation is 450 pages, including 188 figures, 68 tables and a bibliographic list of 525 titles. The main content of the work The Introduction reveals the relevance of the topic, formulates the goals and main tasks of the work, the scientific novelty and practical significance of the results, the main provisions submitted for defense. Chapter 1 outlines general ideas about the main methods for studying the structure (Section 1.1) of regularly organized objects and considers the problems that arise when moving from crystalline structures to structures with reduced dimensionality - liquid crystals (LC) and quasi-two-dimensional films. The appearance of works on the study of the LC structure, when the structural data were obtained by the Fourier transform of the scattered intensity, is associated with the names of B.K. Vainshtein and I.G. Chistyakov. The main research tool was proposed by B.K. Weinstein functions of interatomic distances for systems with macroscopic cylindrical symmetry. This method was further developed with the beginning of the use of the concept of molecular self-folding in the analysis of Paterson maps of a number of polymeric liquid crystal systems and thin anisotropic films. Difficulties arising in the direct determination of the LC structure initiated studies based on a model description of systems with disturbed translational order. In terms of the Hosemann model of a paracrystal, the structure of the main LC phases was considered and their classification was carried out according to the prevailing type of violations of the translational order. The Fonck cluster model can also be considered as one of the options for analyzing systems with various types of disturbances, where a correlation function is introduced to describe local electron density fluctuations, which makes it possible (as in the case of the Hoseman model) to estimate the size of near (roughness) and far (distortion length) disturbances order. In terms of this model, X-ray data for a number of liquid crystal polymers were interpreted. In the last decade, the reflectometry method has been used to study the surface structure and thin flat films. Here, the scattering of a plane wave incident on the interface is considered in terms of the macroscopic refractive index, which characterizes the average properties of the radiation on both sides of the interface. The reflectivity of a flat layer can be calculated using the dynamic matrix method (Parratt algorithm) or in the kinematic approximation (Born approximation). In the case of a layer that is inhomogeneous in density, by introducing macroscopic or microscopic roughness, attempts are made to take into account the existence of transition zones and thus bring the model closer to real systems. Small-angle X-ray patterns obtained for reflection in a reflectometric experiment can be interpreted as ordinary diffraction patterns, which turned out to be very informative in the study of LB films of fatty acid salts, lipid lyomesophases, and lipid-protein systems. However, a large number of reflections during interlayer diffraction is not at all typical for thermotropic liquid crystal systems and LB films formed from mesogenic molecules; therefore, Fourier synthesis does not provide the necessary resolution in these cases, and modeling requires setting a complex electron density profile of the layer. In the diffraction study of liquid-crystal objects, the possibility of their macroscopic orientation is essential: by magnetic and electric fields, tension, shear deformation, flow, the substrate surface, and the free surface of the sample. As a rule, macroscopically uniaxial orientation is set using these methods, and for biaxial orientation, a combination of methods must be used. Highly oriented (single domain) liquid crystal samples can be obtained by heating single crystals. The limitations here may be due to the complexity and often the impossibility of obtaining a single crystal suitable for X-ray photography. Sec. Section 1.2 of the review is devoted to the structure and properties of polar liquid crystals. The reasons for the occurrence of electric polarization Ps in LCs are considered: due to the inhomogeneous orientational deformation of the director field n(r) in the absence of an electric field - the flexoelectric effect, during the process of uniform deformation of the crystal - the piezoelectric effect, and with a temperature change in the spontaneous polarization - the pyroelectric effect. So far, it has not been possible to detect uniaxial LCs with exclusively quadrupole symmetry, which is caused by the instability of the ferroelectric smectic A-phase. However, there are other ways of realizing the polar state in LCs. In the smectic C-phase, the symmetry of smectic layers can be reduced to the m group due to symmetry breaking in the arrangement of heads and rigid perfluorinated tails of achiral molecules or to group 2 due to the use of chiral molecules. The orientational degrees of freedom of the LC are responsible for the transition to the inclined smectic C* phase (according to the phenomenological theory proposed by Pikin and Indenbom), and the polarization is a consequence of the piezoelectric and flexoelectric effects in the LC. Minimization of the free energy of the smectic C with respect to polarization gives a helicoidal distribution of the vector P in the volume, which, in the case of an electric field applied perpendicular to the axis of the helicoid, is oriented in the direction of the field. When the helicoid of the smectic C* is distorted in an external electric field, one should distinguish between a perturbation of the distribution of the azimuthal angle (z, E) - o (z) with a uniform distribution of the angle of inclination of molecules o along the z axis and a periodic perturbation of the angle of inclination of molecules (z, E) = o + 1(z,E) for the unperturbed period ro of the helicoid. Due to the piezoelectric effect, both of these deformations contribute to the macroscopic polarization of the medium. The flexo effect can cause macroscopic polarization of the C* phase only when periodic perturbations of the tilt angle of the molecules under the action of the field occur. The above concepts of the structure and properties of the smectic C (C*) phase implicitly proceeded from the fact that the conformations of molecules do not change during the phase transition, however, the model in which the slope of the aliphatic chains of molecules during the phase transition to Sm–C turns out to be noticeably less than the slope of the rigid central parts, allows us to explain the decrease in Ps with an increase in the length of the alkyl chain due to a decrease in the effective angle of inclination of the molecules. Thus, the ferroelectricity in Sm–C* has an improper nature, and the occurrence of polarization is a consequence of the orientational deformation caused by the tilt of the molecules, the spatial inhomogeneity of the director field, and changes in the conformational state of the LC molecules. The rest of the review (Section 1.3) is devoted to the production and structure of LB films, including the formation and phase states of monolayers at the liquid–gas interface, transfer techniques, film structural types, heteromolecular monolayers and superlattices, and polar films. The latter are important from the perspective of practical application with a focus on their possible pyroelectric or ferroelectric properties and can be formed by the Schaefer method from a highly compressed polar monolayer or from alternating monolayers of various molecules. It should be noted that in both cases the formed film is not required to have a thermodynamically equilibrium structure. Compared to monomeric, polymeric LB films should have a significantly more stable structure. For the cases of polymerization of monolayers at the water-air interface, the influence of the chemical structure of monomeric molecules and the conditions of polymerization on the stability of the monolayer are considered. During the polymerization of LB films or monolayers successively deposited on a substrate, structural changes also depend on many parameters: deposition conditions, the size of the polyreaction zone, the type of the initial structure, and the chemical structure of the monomer. The properties of monolayers formed from polymer molecules depend on the type of polymer, molecular weight, structure of copolymer components, the presence of flexible decouplings, and the conformational state of polymer fragments. Thus, the stability and homogeneity of a monolayer are associated with the spreading of polymer molecules on the subphase surface, which, in turn, depends on the flexibility of the polymer chain and the cohesion of polymer fragments of both the main and side chains. An increase in the length of aliphatic fragments of the side chains (starting from C16) leads to their crystallization. Sec. Section 1.4 is devoted to general ideas about the structure of crown ethers as complexing compounds and their properties in organized systems at the interface. The metal complexes formed during the binding of ions are the more stable, the less the difference between the geometric dimensions of the cations and the cavities of the macrocycles. It should be noted that oxygen macrocycles can also form intramolecular hydrogen bonds with some peripheral proton donor fragment. “Hard” crown ethers (dibenzo-18-crown-6) are characterized by a slight change in the size of the macrocycle cavity and the symmetry of the molecule in metal complexes, and for “flexible” crown ethers (dibenzo-24-crown-8) - conformational diversity. However, when analyzing the processes of complexation, it is expedient to take into account other factors: the nature of the solvent, anion, and substituents in crown ethers. Unsubstituted macrocyclic compounds, as a rule, do not form stable monolayers due to the lack of balance between the hydrophilic and hydrophobic parts of the molecule. In the case of substituted macrocycles, there is no consensus on the mechanism of phase transitions in such systems. The phase transition from the liquid-expanded to the condensed state corresponds to the appearance of an extremum on the isotherm, which should turn into a plateau at lower compression rates. The order of selectivity in monolayers of macrocyclic compounds with respect to the set of complexing ions does not always correspond to that in solutions. The prospect for studying monolayers and LB films of crown ethers is associated with the selectivity of the "guest-host" interaction and the possibility of directed ordering of the resulting system, which can be used to create functionally active film elements. liquid crystal metal complexes. The first rod-shaped lanthanide metallomesogens were synthesized and described by Yu.G. Galyametdinov. X-ray diffraction studies of complexes of the type showed that they have the same structure, at least for the middle part of the elements of the lanthanide group. The nearest environment of a metal atom consists of three oxygen atoms, neutral ligands on Schiff bases, and six oxygen atoms of nitrate groups. The coordination polyhedron is a distorted square antiprism. The mesomorphic properties of lanthanide mesogens depend primarily on such parameters as: the type of complexing metal, the length of the alkyl chains of the ligands, the type of ligand and anion, by varying which it is possible to significantly reduce the phase transition temperatures and the viscosity of the smectic phases of the complexes. The orientational controllability of the mesophase by a magnetic field depends on the magnitude of the magnetic anisotropy of the medium. The orienting torque acting on the LCD in the field ГМ~Н2. Since the values of some lanthanide mesophases exceed the anisotropy of conventional diamagnetic and paramagnetic LCs by several hundred times, orientational effects can be observed in much lower magnetic fields. Previously, studies of lanthanide complexes containing environmental ions of various nature (Cl, NO3, SO4CnH2n+1) were carried out only in the bulk state, but model calculations were not performed, and the temperature behavior with varying field exposure was not studied. The possibility of forming regular film structures from these complexes and their orientational possibilities for controlling the anisotropy of Langmuir layers have not been studied either. Chapter 2 contains descriptions of setups and methods (including computational ones) designed to orientate and study the structure of bulk samples of LC compounds and films formed on their basis. Establishing a correlation between the structural parameters of an object and the mechanism of orienting influence provides additional information about the behavior of its structure under external influences and the possibility of its purposeful modification. Based on these considerations, an apparatus complex was created for structural studies, which makes it possible to orient liquid-crystal compounds in various ways and carry out their X-ray imaging in situ (Sec. 2.1). The complex is based on the URS-2.0 X-ray unit and includes: a magnetic chamber with a temperature cell and a mechanism built into it for stretching polymer samples, a universal URK-3 X-ray camera with attachments designed for it, which allow heating and orienting LC samples by electric fields, flow and continuous shear deformation. The registration of the scattered intensity can be carried out on a flat (or cylindrical) photographic film or with the help of a linear coordinate detector RKD-1, when it is installed instead of a film cassette. The use of solid collimators with round apertures and large base distances provides a sufficiently small beam divergence (no more than 1 10-3), the ability to record large periods (up to 100) and does not require the introduction of collimation corrections. Scattering by Langmuir-Blodgett films was recorded using a KRM-1 X-ray camera with a built-in RKDrazd coordinate detector. 2.2). X-ray photography of the LB films was carried out at fixed positions of the substrate at glancing angles that make it possible to record the diffraction pattern by successively increasing the intensity in each individual reflection. Filtered (Ni filter) CuK radiation was used for X-ray photography. The effects associated with the radiation component with a continuous spectrum were revealed by X-ray photography at various high voltages. In some cases, a combination of Ni and Co filters was used to filter this component. The structure of the LB films was also studied using an EMV-100L transmission electron microscope in the electron diffraction mode and a P4 NT-MDT scanning probe microscope in the atomic force mode. Processing of radiographs and electron diffraction patterns was carried out on an automated densitometric complex, which allows for computer processing of densitograms. The complex is assembled on the basis of an MF-2 microphotometer equipped with a table drive, a displacement scaler, and a recording system from a DP 1M densitometer. The instrumental beam divergence was determined from the width of the reflections of a coarse-grained polycrystalline sample. When taking into account its approximating function, the Gaussian function was used. When considering the structure of liquid-crystal compounds, paracrystalline violations g1 (violations of long-range order) and the sizes of coherent scattering regions were calculated from the radial diffraction width of reflections. The degree of orientation S and the average values of the corresponding scatter angles of the layered structure (mosaic) and molecules in the sample were estimated from the azimuthal smearing of small-angle and wide-angle reflections I(), respectively. Preliminary information about the structure of the molecules under study (Section 2.4) is very important in structural studies of complex chemical compounds. The search for an energetically favorable conformation of molecules was carried out using computer simulation: the MM+ method, geometric optimization. The interpretation of the data of small-angle X-ray scattering by smectic layers or LB layers of a film formed on the basis of mesogenic molecules was carried out using structural modeling (Section 2.5). Modeling of the layered structure began with the alignment of the structure-forming fragment of the layer from the molecules constructed in the molecular modeling program and the formation of an array of atomic coordinates that determine the electron density in the cross section of the layer. The projection of atomic coordinates onto the normal to the layer plane is used to calculate the structural amplitude of the layer and scattering by the multilayer system within the framework of a one-dimensional model. The structural amplitude of the layer F(Z) is calculated by the formula where fj and zj are the amplitudes and coordinates of the atoms of the structure-forming fragment of the layer, respectively, and Z is the coordinate in the scattering space. The intensity I(Z) of scattering by a multilayer system is calculated as where dz is the layer thickness and M is the number of layers. The layer thickness was set equal to the interlayer diffraction period obtained from the X-ray experiment. The main fitting parameters in modeling are the slope of molecules in a layer and the overlap of their end fragments in adjacent layers. In reality, there are more parameters, since in the general case it is necessary to set the azimuthal orientation of the molecules when tilted and, within the allowable range, vary their conformation. The fit criteria are the reproducibility of the ratios of the intensity of multiple reflections obtained in the experiment and the minimum R-factor. When compared with the experiment, the calculated intensity is modified taking into account the geometry of X-ray photography, polarization, absorption, and mosaicity of the sample. In the case of bulk smectic structures, the azimuthal intensity distribution, which depends on the degree of sample orientation, is taken into account. In addition, it is necessary to take into account the intensity pumped into the background (the influence of the temperature factor). To do this (after preliminary subtraction of the intensity scattered by air), the ratios of the intensities in the discrete peaks and the background below them are estimated, and then the corresponding fractions of the background intensity are subtracted from the integral intensity of the calculated maxima. The electron density (its projection onto the normal to the layer plane) is needed only to track the dynamics of changes in the diffraction pattern when varying the fitting parameters. The calculation uses the number of electrons in each atom of the structure-forming fragment and the corresponding atomic radii. To study the behavior of molecular layers on the water-air interface and design multilayer films on their basis, an automated LB setup was designed (Section 2.6), which makes it possible to form molecular layers on the water surface at different temperatures and in the presence of a magnetic field, to monitor their state and transfer the formed layers onto solid substrates (silicon or collodion) by various methods. The unit can operate in one- and two-tray modes with two- and one-barrier compression of the floating layer and maintain its pressure during film deposition on the substrate. The dependence of pressure on the area per molecule (-A isotherm) is displayed on the display screen in real time with saving the created file. In the formation of monolayers, in all cases, the initial coverage factor was less than unity. Chloroform, benzene, and heptane were used as solvents. The working concentration of solutions is 0.2-0.5 mg / ml. Compression began after the solvent had evaporated (after 30 minutes). The movement of the barrier at speeds of 3–5 mm/min in most cases made it possible to implement a quasi-static mode of compression of the floating layers. Chapter 3 presents the results of X-ray diffraction studies of chiral CH2=CH-COO-CH2-C*(CH3)H-(CH2)2-COO-(C6H4)2-O-R and achiral CH2=CH-COO-(CH2)6 -О-С6Н6-СОО-С6Н6-О-R` LC monomers (M), their mixtures (MIX), as well as homo- (P) and copolymers (CPL) based on them in various phase states with a projection on polar properties in depending on the molecular structure and composition, Table. one. Indexing of X-ray diffraction patterns with subsequent analysis of reflection extinctions and access to the space group allows us to conclude that the chiral monomers M1 and M2 form smectogenic crystal structures that can be described in terms of the monoclinic syngony with the symmetry of the space group P21. In all cases, head-tail packing of molecules is realized both in the layer and from layer to layer, however, only in the structure of the chiral monomer M2 (a=9.89, b=8.84, c=34.4, =125, 7o, n=4, =1.315 g/cm3), parallel orientation of transverse dipole moments (m2.5 D) is realized. The chiral monomer M has packing with 2-layer periodicity (a=5.40, b=8.36, c=56.6, =112.4o, n=4, =1.311 g/cm3), where the dipole moments of the molecules (m4.7 D), are compensated due to the formation of dimers. Schemes of phase transformations of monomers and homo- and copolymers based on them 5 and the slope of the molecules in the layers is 26°. A decrease in the tilt of the molecules facilitates the azimuthal mismatch, which contributes to the transformation of the bilayer structure into a single layer. Dimers in the SmF* phase are not destroyed, and therefore the compensation of dipole moments is also preserved. In M2, the azimuthal mismatch and the occurrence of radial disturbances are restrained due to additional dipole-dipole interactions, therefore, during melting, a Cr-H* phase is formed (a=4.53, b=9.18, c=34.5, =117.1o, n=2, =1, g/cm3) with the same P21 symmetry. There is no compensation for transverse dipole moments of molecules in the layer of the Cr-H* phase. Achiral monomers M3 and M4 in the crystalline phase form monoclinic structures of the smectogenic type with polar symmetry: cm3) and P2 for M (a=16.0, b=4.96, c=37.2, =113o, n=4, =1.246 g/cm3). Space group P21 requires antiparallel longitudinal and parallel transverse orientation of the axes of M3 molecules, and group P2 requires pairwise antiparallel orientation and longitudinal and transverse axes of M4 molecules. Due to the misorientation of the dipole moments of the C=O groups, the molecules M3 and M have a total transverse dipole moment m 1 D. When heated, M3 forms SmC and N, and M4 forms SmA and N mesophases. For M3 in the nematic, the ratio of the parameters of disturbances in the longitudinal and lateral stacking indicates that the layered structure has not been completely destroyed. In the M4 nematic phase, the situation is reversed, which is typical of the classical nematic phase. In mixed compositions of chiral and achiral molecules in the range of studied concentrations (Table 1), phase separation in the crystalline state is always observed, while in the mesomorphic state it depends on the structure and ratios of the mixed components. Thus, with a decrease in the difference in the lengths of the mixed molecules, the tendency to phase separation increases. However, with respect to the influence of the concentration of chiral components M1 and M2 in mixtures with the achiral component M3 on the phase separation, the situation is mutually opposite. Strengthening of the tendency to phase separation with increasing concentration of M1 is associated with the formation of relatively stable dimers, which reduces their ability to mix. In the studied mixtures, one should not expect stronger polar properties than in the initial components. Chiral homopolymers P1 and P2 obtained by free radical polymerization from monomers M1 and M2 form SmF* and SmC* phases with a bilayer structure. From the point of view of the best agreement with the X-ray experiment, it follows that the side groups are inclined to the main chain and oriented so that the C-CH3 fragments in them lie in the plane of inclination of the side groups. In this case, the dipole moments of the C=O groups in the layers of the bilayer turn out to be identically oriented perpendicular to the tilt plane. Such a model is also confirmed by the energy assessment in computer simulation of the structure of P1 and P2 molecules. X-ray diffraction patterns of polymers oriented by magnetic (1.2 T) and constant electric (700 kV/m) fields are typical for chiral smectics, but the structural parameters estimated from them have some differences due to the difference in the orientational mechanism. Smectic layers are oriented perpendicular to the magnetic field and along the electric field. The effect of an electric field on the translational ordering of a layered and intralayer structure is, on the whole, weaker than that of a magnetic field. Helicoid spinup is not observed. Achiral homopolymers P3 and P4. X-ray diffraction studies show that polymer P3 forms three SmA structures with commensurate 59.5 and incommensurate 54 and 47.5 bilayer periods. The structural transformations of SmA-SmAd1 and SmAd1-SmAd2 seem to be based on effects associated with both changes in the flexibility of the interchanges connecting mesogenic groups with the main chain and changes in the flexibility of the main chain. P3 managed to orient only by twisting and stretching. In this case, the influence of the orienting effect on the polymer structure was found, which manifests itself in a change in the layer period (twisting) and intralayer disturbances (twisting, stretching) compared to an unoriented sample. Polymer P4 with an additional C=O fragment in the tail of the side groups forms two smectic phases, SmF and SmC. Since the transverse dipole moments of the side groups in P4 are less than D, the prognosis for the detection of strong polar properties in this polymer is negative. Copolymers based on monomers M1 and M3. X-ray diffraction patterns corresponding to the Sm*F and Sm*C phases were obtained from copolymers oriented by a magnetic field, but differing in the azimuthal intensity distribution in reflections depending on the ratio of chiral and achiral components. In CPL1-375, the X-ray patterns in both phases correspond to the so-called bookshelf structure, in CPL1-350 they are typical of the mentioned chiral smectic phases, and the X-ray patterns of CPL1-325 are characteristic of a chevron-type structure. When oriented by a constant electric field, no such differences are observed. Due to the different orientation mechanism, the electrically and magnetically oriented copolymers (as well as the P1 homopolymer) have different structural parameters. Modeling of the bilayer structure of copolymers and diffraction calculations make it possible to explain these differences. So, in CPL1-375 and CPL1-325, the layers that make up the bilayer have a different composition in terms of the ratio of chiral and achiral components, that is, one layer contains mainly the P1 or P3 component, respectively, and in the other, the ratio of components is almost the same. In the first case, this apparently led to a certain increase in the pitch of the helix, and in the second, to the destruction of the helicoidal structure. In CPL1-350, the composition of both layers of the bilayer is the same, and only in it the degree of orientation of the side groups when exposed to an electric field is higher than in the case of a magnetic field. And this is a sign of deformation of the helicoidal structure, leading to macroscopic polarization of the copolymer. From the energy estimate of the CPL1-350 fragments with different orientations of the side groups, it follows that the fragment with the lowest energy has the following characteristics: the same ratio of chiral and achiral side groups in the layers of the bilayer, the opposite azimuthal orientation of both of them in neighboring layers, and the slope of the side groups. groups to the main chain. Such a structure of the fragment does not conflict with the diffraction-confirmed model. In this case, the polarization in the layers of the bilayer must have the same direction. It should be noted that the energy difference between polar states with different azimuthal orientations of chiral groups relative to the main chain for the CPL1-350 fragment is smaller than for CPL1-375 or P1, which makes it possible to switch the structure by a smaller electric field. Copolymers based on M1 and M4 monomers form bilayer SmF* and SmC* phases. For copolymers with different ratios of chiral and achiral achiral components, characteristic temperature changes in the structural parameters inside the SmC* phase are observed, apparently due to different contents of chiral and achiral side groups in the bilayer layers (the situation is the same as in the case of copolymers based on M1 and M3). That is, the CPL1-475 and CPL1-425 bilayers can be considered as a kind of two-phase system. In the case of CPL1-, the prospects for detecting polar properties are the same as for CPL1-350, but due to interactions of ester groups in the tails of achiral side fragments, the structure of the copolymer is less labile. A distinctive feature of copolymers based on M2 and M monomers is a relatively high SmF*-SmC* transition temperature and a significantly smaller slope angle of mesogenic groups in the SmC* than in the SmF* phase, which facilitates the azimuthal mismatch. The bilayer structure of CPL2-375 consists of layers of the same composition with partial compensation of the dipole moments of the chiral component. The CPL2-350 does not have this compensation (it has the same structure as the CPL1-350), and the polarization must be stronger. Due to the smaller (compared to CPL1-350) transverse dipole moment, the CPL2-350 structure is more conservative in terms of the possibility of electrical switching. The most probable CPL2-325 model: in the SmF* phase, bilayer layers of different composition but with the same polarization direction; in the SmC* phase, the polar properties become weaker due to the azimuthal detuning, while in the SmA phase, the structure becomes nonpolar due to the complete azimuthal misorientation of the side groups. Macroscopic polarization in SmF* and SmC* can appear only upon deformation, but due to the relatively small amount of the chiral component, the effect cannot be strong. Chapter 4 is devoted to the production of polar Langmuir-Blodgett films and the stabilization of their structure by photopolymerization. The instability of artificially built film structures leads to a violation in one form or another of their regularity and even integrity and, as a result, to a partial or complete loss of properties that ensure the performance of the main function. Parasubstituted chiral biphenyls M1, M2, achiral phenylbenzoates M3, M4, and their mixtures, studied in the bulk state (Chapter 3), served as the starting material. The compounds contain an acrylate group, which made it possible to polymerize them in a monolayer on the surface of water and in a multilayer film on a solid substrate using UV radiation from a mercury lamp. The characteristic -A isotherms obtained during the formation of monolayers of monomers are shown in Figs. 1. All molecules have a hydrophobic tail and a hydrophilic head, but the presence of other hydrophilic and hydrophobic groups in the molecules does not allow them to be classified as classical amphiphilic compounds. From the ratios of the areas per molecule in the condensed phase and the cross sections of the molecules, it can be concluded that all monomers form monolayers, the molecules in which are arranged obliquely relative to the water surface. The density and stability (determined by the pressure of destruction - collapse) of monolayers is higher for biphenyls than for phenylbenzoates, and they increase with increasing length of the hydrophobic tail of the molecules. The stability of monolayers formed by mixtures of biphenyls and phenylbenzoates (M1-M3, M2-M3) depends on their ratio. The greatest positive effect is achieved at high concentrations of biphenyls (75%) M1 or M2. At high concentrations, M3 is the worst indicator. And isotherms for monomeric monolayers make it possible to choose rational photopolymerization conditions. Under UV irradiation of monomeric monolayers, in all cases, with the exception of the monolayer of the M3 monomer, their shrinkage is observed (a decrease in the area per molecule, leading to a sharp drop in pressure) (Fig. 1). UV polymerization of homomolecular monolayers does not always lead to an increase in their stability, for example, in the case of monolayers M2 (decrease in stability) and M3 (a very slow increase in pressure indicates the destruction of the monolayer during compression). Rice. 1. -A isotherms of floating layers based on: a - M1 and P1; b - M3 and P3: (1) monomeric, (2) monomeric after UV irradiation, and (3) polymeric. %) exceeds the stability of the initial monomeric monolayers. Monolayers formed on the basis of molecules of the comb-like polymer P1 (based on monomer M1) are more stable than monomeric ones, but all attempts to detect the regular multilayer structure obtained on their basis on a solid substrate by X-ray method were unsuccessful. To determine the position of the side groups of the polymer in the polymer monolayer, a complex lattice (superlattice) was created, which is an LB film of alternating monolayers of polymer P and lead stearate, which play the role of structuring spacers (Fig. 2). Comparison of small-angle X-ray diffraction patterns obtained from such a superlattice and from a multilayer LB film of lead stearate made it possible to establish that the side groups of the polymer basically almost lie in the plane of the film, and, consequently, on the water surface. The lack of layer regularity in the polymer film is caused by the non-smoothness of the surface of the floating layer due to the impossibility of laying the main chain into a two-dimensional coil on the water surface. Rice. Fig. 2. Small-angle diffraction patterns of the LB film of lead stearate (a) and the superlattice assembled from monolayers of polymer P1 and lead stearate (b), the model of the superlattice and the calculated diffraction from it (right). Thus, there are two ways to solve the problem of obtaining regular polymeric LB films: 1 - through UV polymerization of monomeric multilayer films on a solid substrate and 2 - through building a multilayer structure from UV polymerized floating monolayers. The multilayer film of the M1 monomer fabricated according to Schaefer has a polar bilayer structure with the orientation of molecules in layers of the same type as the side groups of the polymer P1. The reason for the appearance of a structure with bilayer periodicity is the reactive deposition of a second monolayer or the expulsion of some molecules from a layer on a substrate with a head-to-head flip. UV irradiation of the M1 film leads to an increase in its periodicity by almost 1.5 times, due to the occurrence of defects in the form of kinks during the formation of the polymer chain, which should reduce its polar properties. The LB film formed according to Schaefer from M1 monolayers UV polymerized on water gives a diffraction pattern corresponding to a bilayer structure very close to the structure of polymer P1 in the smectic F phase. Here, the simulation makes it possible to distinguish the bilayer structure resulting from the reactive deposition of the second isotactic polymer monolayer (one-sided comb) on the substrate from the bilayer structure of the syndiotactic polymer (double-sided comb), Fig. 3. Since for the second variant the mismatch factor (R-factor) is significantly lower, it can be concluded that the isotacticsindiotactics undergo a conformational transformation in the monolayer when it is separated from water. Rice. Fig. 3. Structural models of LB films from UV polymerized monolayers based on M1 monomer and the corresponding interlayer diffraction curves: a) for isotactic molecules (R = 0.335) and b) for syndiotactic molecules (R = 0.091%). LB films of M2, M3, and M4 monomers have a structure with a single-layer periodicity, but in contrast to the crystalline phase with a parallel arrangement of molecules in layers. From the monolayers of monomer M3 at various pressures, structures were obtained that, in terms of interlayer periods, were close to the crystalline and smectic C phases. This indicates that the condensed phase of the monolayer also includes a two-dimensional analogue of the liquid crystal phase. A characteristic feature of M2, M3, and M4 monomeric films is the overlap of end groups in neighboring layers, which can screen C=C bonds and prevent polymerization. Thus, UV irradiation of LB films of M3 and M4 monomers does not lead to any structural changes in the film due to the screening effect. The structure of the films fabricated from UV polymerized M2 and M4 monolayers also has a single-layer periodicity, and not a bilayer one, as in a comb-like polymer in a smectic phase. The interaction of ether groups in the tails of the M2 and M4 molecules apparently prevents the conformational transformation with the formation of a bilayer structure. It was not possible to build a regular multilayer film from UV-irradiated M3 monolayers (as in the case of a mixture with 75% M3 content) due to their inhomogeneity. There is no phase separation in LB films of M1-M3 and M2-M3 mixtures (with the exception of MIX1-375). All films have a structure with a single-layer periodicity and with a parallel arrangement of molecules in layers. In the structures of LB films of mixtures (with the exception of the MIX2-375 mixture), there is an element of overlapping of the end groups of molecules in neighboring layers, which prevents UV polymerization of the film. This conclusion can be confirmed by changes in the UV-irradiated LB film of the MIX1-375 mixture that occurred after 1.5 years. One of the heterophase structures with a single-layer periodicity was transformed into a bilayer structure with a period coinciding with the period of the crystalline phase of the M1 monomer. An electron diffraction study of a LB film based on UV polymerized MIX1-350 monolayers shows that the film contains mainly a monomeric component. Simulation of the film structure and calculation of X-ray diffraction confirm this. Based on the results obtained, it can be concluded that after UV irradiation the resistance of monolayers decreases due to their heterophasic nature. Monolayers along with the polymer component may contain a significant amount of monomer. And since the polymer side groups due to the arising steric hindrances almost lie on the surface of the water, when the substrate contacts the film during the Schaefer transfer, monomeric molecules can predominantly sit on it. In the film based on UV polymerized MIX1-375 monolayers, the monomeric component is also present, but in an insignificant amount. Modeling and diffraction calculations give a polar structure of isotactic polymer molecules with single-layer periodicity. Thus, an increase in the concentration of the phenyl benzoate component in the mixture leads to the formation of a looser monolayer and, as a consequence, to a more pronounced heterophasity after UV polymerization. Chapter 5 presents the results of studies on the formation of structures with transport channels from the cavities of macrocyclic molecules (crown ethers) in combination with the possibility of controlling their macroscopic orientation in Langmuir monolayers and LB films and the possibility of stabilizing the structure of the latter. Bulk samples of dibenzo-18-crown-6 and dibenzo-24-crown-8 with various substituents containing azomethine and enaminoketone fragments (Fig. 4) and LB films based on them, including conductive films formed on based on complexes of crown ethers with potassium undecylenate (KO-CO-(CH2)9=CH2), sodium laurate (Na-O-CO-C11H23) and C60 fullerene. Bulk samples of disubstituted crown ethers in the crystalline phase form structures belonging to the monoclinic system with the same symmetry P2/m. The structures are close in packing density, where there is a common element - overlap packing, in which the substituents of neighboring molecules overlap, which is typical for nematogenous structures (Fig. 5). The cell parameters depend on the size of the crown and the length of the lateral substituents, which also affect the degree of extension of the central fragment. The presence of enaminoketone groups in the substituents leads to a significant increase in the transverse dimensions of the cell due to the number of its constituent molecules. The reason lies, apparently, in the formation of not only intramolecular, but also intermolecular N-H···O hydrogen bonds during the implementation of pair contacts of enaminoketone fragments of neighboring molecules, which makes the structure energetically more favorable. The presence of such bonds is indirectly confirmed by the data of the IR spectra of these compounds, where there is a wide and intense absorption band of stretching vibrations of N-H groups in the region of 3416 cm-1 (usually this band has a low intensity). When such a lattice melts, two-dimensional fragments of molecules crosslinked by hydrogen bonds remain. Since the longitudinal disturbances in the packing of these fragments are smaller than the transverse ones, a structure with signs of layering appears. Indeed, the X-ray diffraction pattern obtained by melting the sample in a magnetic field corresponds to a nematic, but with signs of a chevron structure. This is the so-called oblique cibotactic nematic phase. When crown ether molecules interact with azomethine fragments, there are no hydrogen bonds in the substituents and, as a result, a classical nematic phase is formed upon melting of the crystal lattice. Due to hydrogen bonds, the structure becomes more conservative, and this factor can be used to stabilize the layered structures formed by the LB technology. Formation of monolayers and structure of LB films. The isotherms obtained during the formation of Langmuir monolayers based on molecules of disubstituted crown ethers -A may differ in shape and onset of pressure growth. The difference in their course, as it turned out, depends not only on the degree of coverage or concentration of dissolved molecules, but also, to a decisive extent, on the temperature of the subphase. It has been established that at temperatures below 17 -A, the isotherms have a characteristic hump or plateau, the position of which is not strictly fixed both in terms of area and surface pressure. The presence of a hump (or plateau) on the -A isotherms of crown ethers is usually associated with a phase transition from a liquid-expanded to a condensed state, although there is no unambiguous opinion regarding the mechanism of the phase transition. The type of phase transition is determined by kinetic limitations: with a decrease in the compression rate or a decrease in the length of substituents, the hump turns into a plateau. With an increase in temperature, a degeneration of the hump (or plateau) is observed, and, starting from 23C, it is no longer observed, Fig. 6. Taking into account all the revealed features of the behavior of -A isotherms, the mechanism of structural transformations in the floating layer can be explained as follows. Crown ether molecules are prone to aggregation, but this can be prevented by solvent molecules held by crown ether molecules. The ratio of aggregated and non-aggregated molecules in the formed layer will determine the position of the hump or plateau (phase transition) on the isotherm. When a certain pressure (depending on temperature) is reached, solvent molecules are squeezed out of the monolayer and the mechanism of aggregation of flat-lying crown ether molecules is triggered. This interpretation is also supported by the fact that only a smooth isotherm is obtained during the secondary compression of the expanded monolayer, since the formed aggregates no longer decompose. At an elevated temperature (23-24°C), the solvent begins to leave the water surface already at the initial stage of monolayer formation and, as a result, a smooth isotherm is obtained. Depending on the conformational rigidity of the crown ethers, during the phase transition, the molecules either change their spatial orientation, colliding with each other and then flipping on an edge (hard crown-6), or twist in the crown region, due to which the tight contact of neighboring molecules during the formation of an aggregate implemented with their longitudinal shift relative to each other (flexible crown-8). This causes the difference in the structure of the formed monolayers and, as a consequence, in the structure of the LB films obtained on their basis. According to X-ray data, they respectively have either a quasi-two-dimensional structure with a single-layer periodicity or an incommensurate bilayer structure with an internal overlap of molecules. Rice. 6. -A crown-6-a10 isotherms: Fig. Fig. 7. Packing of crown-8-e12 molecules in LB film, a – 0.5 mg/ml; 1.7 ml/m2; 17оС, electron density (z), experimental (1) b – 0.5 mg/ml; 1.7 ml/m2; 24оС, and calculated (2) scattering intensity в – 0.25 mg/ml; 2.14 ml/m2; 17оС. multilayer structure for LB films. When forming LB films from floating layers of disubstituted crown ethers, the structure of substituents can have a significant effect on the stability of their structure. Thus, in the structure of LB films of crown ethers with azomethine groups in substituents, a significant overlap of terminal fragments of molecules in neighboring layers occurs, which does not allow us to consider such a structure as a quasi-two-dimensional structure. Such a structural element is characteristic of the crystalline phase. In the case when the substituents contain enaminoketone groups, the structure of the LB films remains either quasi-two-dimensional, similar to a smectic structure with a single-layer structure (crown-6e-n), or incommensurate bilayer structure (crown-8e-n, see Fig. 7) periodicity. Apparently, the interaction of active enaminoketone groups of neighboring molecules in layers either directly or through a chloroform molecule with the formation of hydrogen bonds makes the quasi-two-dimensional structure more stable with respect to crystallization. The study of the behavior of crown ether molecules in floating layers together with salts of fatty acids and C60 fullerene was carried out in order to create film structures with spatially localized nanosized conducting elements. Isotherms of floating layers based on mixtures of crown-8-e12 or with potassium undecylenate (UK) or sodium laurate (LN) in the ratio 1: differ from the isotherm of pure crown-8-e12 by a phase transition shift (in the form of a hump) from liquid expanded to liquid-condensed state into the region of large areas per molecule, which indicates the formation of complexes. Their behavior in a monolayer is very similar to the behavior of hard crown ether molecules, since the formation of a complex causes the crown ether crown to lose its conformational mobility. The second phase transition (in the form of a plateau or inflection), associated with the reorientation of the fragments of the formed complex in the floating layer, like the first one (in the form of a hump) depends on temperature, but to a lesser extent. At 24°C, the extent of the plateau only decreases and shifts to a region of smaller areas per molecule, while the hump disappears altogether. According to the data of the LB X-ray experiment, the film of the CE-UK complex deposited from the condensed phase has a quasi-two-dimensional structure with a single-layer periodicity (the central parts of the CE molecules are edge-tipped, and there is no overlap of the end fragments). There are two ions (K+) in the cavity of the crown ether (donor), and the acidic residues are built into the layers and oriented parallel to the substituents, Fig. 7. Accounting for the regular incorporation of solvent molecules into the model structure leads to a decrease in the R-factor from 0.038 to 0.024. The structure of the LB film based on the complex formed by crown-8-e12 with LN differs in the arrangement of acid residues (not along, but across the substituents). The LB films of the CE-UK and CE-LN complexes are quasi-two-dimensional and do not crystallize. A separate film layer can be considered as a sandwich structure consisting of a conductive layer containing conductive channels formed by CE coronas and dielectric layers formed by CE substituents. In general, the film is a package of such sandwiches, which can serve as a prototype of a nanoscale multicore cable with insulated wires, Fig. 8. Crown ethers were also used to suppress the aggregation of C60 fullerene, which is prone to the formation of three-dimensional aggregates, which makes it very problematic to form Langmuir monolayers and regular layered structures only on its basis. The use of an unsubstituted crown ether as a complexing agent capable of forming a stable monolayer despite the absence of a hydrophobic hydrophilic balance is expedient for increasing the area on the subphase surface coming to the macrocycle cavities, and, consequently, the probability of fullerene molecules getting into them. An important feature of the -A isotherms obtained in the study of structural transformations in the floating layers of DB18C6 and C60 (with a ratio of 2: 1) is the fact that the beginning of the pressure increase corresponds to an area significantly exceeding the maximum area per conventional molecule, which indicates on the absence of aggregation of C60 molecules at the initial stage of monolayer formation. Structural transformations in the monolayer, which result in the formation of sandwich-type complexes, are shown in Fig. 9. A small hysteresis in the course of the forward and reverse isotherms also indicates that C60 aggregation is largely suppressed, since the crown-ether-fullerene complex is formed due to steric hindrance and decompresses during decompression. Rice. Fig. 9. -A isotherms and structural diagram. 10. Structural model and projection of tour transformations in the floating electron density of the layer, experimental layer based on DB18C6 and C60. dimensional (1) and calculated (2) diffraction 11. Model structure and AFM image of a LB film based on complexes formed by DB18C6 and C60 molecules. The data of small-angle X-ray diffraction (Fig. 10) and AFM study (Fig. 11) of the LB film assembled from heteromolecular monolayers of DB18C6 and C60 showed that the sandwich complex is the basic element in the layer structure. In this case, the structure is such that the Cs are in contact with each other, forming chains that do not go beyond the boundaries of a separate layer. It should be noted that the obtained LB films (as well as films based on the CE-UK and CE-LN complexes) are uniaxial and do not have a macroscopic orientation in the plane of the layers. Chapter 6 Here are the results of structural studies of bulk samples and LB films of mesogenic lanthanide complexes, which attract attention both for their magnetic properties (strong organic paramagnets) and significantly lower (compared to complexes containing anions of a different nature) phase transition temperatures, Table 1. 2. The main attention was paid to the temperature behavior of the structural parameters of the bulk phases of the complexes upon orientation by a magnetic (or electric) field, the establishment of a correlation between the structure of these phases and the structure of LB films formed on the basis of the complexes, and the possibility of using these complexes to create biaxial film textures. Structural formulas of lanthanide complexes and their magnetic anisotropy С6Н3(ОН)-С=N-С18Н37 - Tb [X]3 SO4-C12H25 С14Н29-О-С6Н3(ОН)-С=N-С18Н37 - Bulk samples were oriented in an X-ray magnetic chamber by a field of 1.2 T as in a fast ( 1 deg./min.), and with slow (0, deg./min.) cooling from the isotropic phase. X-ray imaging of oriented samples was carried out in situ in a heating cycle in the range from room temperature to the clearing point. The studied complexes form two (SmF and SmС) or three (SmB, SmF and SmС) smectic phases. In complexes with shorter ligands (Dy and ErI complexes), the SmB phase is not observed, apparently due to the fact that the SmF–SmB phase transition temperature for them is below the glass transition temperature. A feature of oriented samples is a weak orientation as a whole at a sufficiently high degree of orientation of the layer structure itself (S = 0.8). In this case, as shown by diffraction calculations from models, the molecules of the complexes have an elongated conformation, but in the SmС phase there is a tendency to a slight overlap of the terminal fragments of the ligands in neighboring layers. The behavior of the diffraction parameters of complexes during phase transitions strongly depends both on their molecular structure and on their prehistory - on the cooling rate of the samples during field orientation and on the nature of the field (electric or magnetic). The cooling rate in a magnetic field affects the SmF-SmC phase transition temperature. However, if the shift of the phase transition towards a lower temperature at a higher cooling rate observed in the Ho complex can be explained by the effect of supercooling, then in the Dy complex this shift occurs towards a higher temperature. Another unusual fact for this complex oriented during slow cooling in a magnetic field is a significant shift in temperature of the characteristic changes in the width of small-angle and wide-angle reflections (Fig. 12). That is, the dysprosium complex behaves as a two-phase system: the central parts of the complex, which form the layers, are one phase, and the ligand tails, which form a kind of interlayer between the layers, are another phase. Moreover, the two-phase nature manifests itself as the effect of a magnetic field, in which the central part of the complex (paramagnet with negative magnetic anisotropy) and the ligand tails (with positive diamagnetic anisotropy) should be oriented differently. Upon rapid cooling in the field, no effect is observed, since in this case the molecule of the complex behaves as a single whole. In the case of erbium complexes with positive magnetic anisotropy (Table 2), the characteristic changes in the width of reflections during the phase transition occur synchronously, as in a single-phase system, since there is no conflict associated with the orientation of the central part of the complex and the peripheral groups of ligands in a magnetic field (Fig. 12 ). Rice. 12 Temperature dependences of the half-widths of the wide-angle () and small-angle () maxima of the Dy (left) and ErII (right) complexes. Orientation during slow (,) and fast (,) cooling in a magnetic field of 1.2 T. When the Dy complex is oriented by a constant electric field in the SmC phase, there is a tendency to a noticeable decrease in the layer period, and in the low-temperature phase the layer period coincides with the length of the molecule, as in the SmB phase. In this case, no noticeable changes in the width of small-angle reflections during the phase transition are observed, and the width of wide-angle reflections continues to increase significantly after the phase transition. The reason is the orientation mechanism. In a constant electric field, the molecules of a complex with positive dielectric anisotropy tend to orient themselves parallel to the field. In the SmC phase, due to the significantly increased conductivity, which is maximum along the layers, there is a tendency for them to turn along the field. It is the orientational conflict that leads to an increase in the slope of the molecules in the layer. X-ray imaging of the complexes upon cooling down to –15°C showed that they do not crystallize, but retain the smectic structure with structured layers (SmF or SmB) in the vitrified state. Based on this fact, it can be expected that the multilayer structure of LB films will be conservative to the same extent. And the isotherms obtained during the formation of Langmuir layers based on lanthanide complexes are of the same type, fig. 13. They are characterized by zero initial pressure and have a number of inflections, indicating the complex nature of the structural-phase transformations in the floating layer, due to a change in the conformation of the complexes, which varies from elongated (in the liquid-expanded phase) to very strongly bent (in the condensed phase). The first plateau on the isotherm corresponds to the transformation of a condensed monolayer into a bilayer, and the second one corresponds to structural transformations associated with a change in the conformation of complexes in the upper layer of the bilayer structure from bent again to elongated (in this case, the molecules stand on their tails). An increase in the temperature of the subphase or the rate of compression of the monolayer leads to the degeneration of the plateau and the shift of phase transitions towards larger areas per molecule. In these cases, the floating layer becomes less stable due to greater heterogeneity. Subsequent studies of LB films based on complexes showed that their structure depends on the deposition pressure, Table 1. 3. At low transfer pressures (up to a plateau), smect-like structures with a shorter period (large molecular slope) are formed than at higher pressures (above the first plateau), when the structure of the LB film is very close to the structure of low-temperature smectic in a bulk sample. At pressures above the second plateau, structures of different types can exist in the floating layer due to its inhomogeneity, Table 1. 3. The ability of a liquid crystal structure to respond to a magnetic field has been used to create macroscopically more ordered thin films of lanthanide complexes than standard LB technology suggests. When a magnetic field is included in the formation of a floating layer (Fig. 11), it becomes possible to obtain film structures with a biaxial texture. The designed magnetic attachment allows you to create a field with induction B=0.05 T (H=4·104 A/m). As the calculation of the Freedericksz critical field (Hc=2·102 A/m) shows, this is sufficient for the orientation of mesogenic complexes on the surface of the subphase. Transfer pressure and structural data for LB films of the Dy complex. Reflex d, I, rel. units Reflex d, I, rel. units Reflex d, I, rel. units During the formation of Langmuir layers based on complexes in the presence of a magnetic field, a number of characteristic differences appear on the isotherms, fig. 15. This is a later onset of pressure buildup at the initial fig. 14. The configuration of the magnetic field in fig. 15. -A isotherms of the Tb complex, projections onto the bath LB plane. 1 - obtained during the formation of monolayers of the bath wall, 2 - barrier, 3 - plate without a field (a) and in the presence of a magnetic stage of monolayer formation, a decrease in the length of section 1-2, corresponding to the gas phase of the monolayer, a faster increase in pressure after the transition to the liquid-expanded phase (segment 2-3), a shift towards smaller areas of characteristic inflections or plateaus on the isotherms in the region of the condensed state (section 3-4 on the isotherm corresponds to the 1st condensed phase, and 4-5 corresponds to the stage of bilayer formation). Here the effect of the ordering of molecules in the field affects - the packing becomes denser. The magnetic field effect also manifests itself in the structure of LB films. For example, in films of Dy and Tb complexes obtained at low (6 mN/m) pressure, the interlayer periods noticeably increase and become equal to the period of films obtained at high (19 mN/m) pressure. At the same time, the electron diffraction experiment indicates the appearance of a texture in the film plane, Fig. 16-b. However, a biaxial film can only be obtained by depositing monolayers at a relatively low pressure (mN/m). The reason lies in the conformational relaxation of molecules. At high pressure, the molecules of the complex in the monolayer are strongly bent, and when separated from the water surface, they straighten out with the destruction of the azimuthal orientation specified by the field. At low pressure, molecules are slightly bent, and conformational relaxation is not so catastrophic for azimuthal orientation. A biaxial texture in a film can also be obtained using the guest-host effect. The situation when guest molecules at the stage of formation of a floating monolayer in the presence of a magnetic field were oriented by the molecules of the complex was implemented to obtain ultrathin films with planar anisotropy in various systems. So, on the basis of heteromolecular floating layers of a mixture of ErII complex - tetrasubstituted porphyrin with a molar concentration of 1:2.4, respectively, optically anisotropic LB films with a sufficiently high anisotropy (orientation degree S = 0.84) were obtained. In this system, the molecules of the complex oriented not the individual porphyrin molecules, but their aggregates, as indicated by the appearance of a plateau in the initial region of the -A isotherm, which is otherwise very similar to the isotherm of the ErII complex. To create LB films with a given anisotropy of planar conductivity, the ternary system crown ether - sodium laurate - terbium complex was used (the molar ratio varied from 1:2:1 to 100:200:1, respectively). The compatibility of all molecules in the overall structure was based on the fact that both the crown ether–sodium laurate mixture and the terbium complex (previously studied) form inclined quasiwww.sp-department.ru two-dimensional layered structures with not too different periods in the LB film. The negative magnetic anisotropy of the molecules of the terbium complex leads to the fact that the molecules in the floating layer are oriented perpendicular to the magnetic field, forcing the anisometric crown ether molecules to orient in the same way. The orientation of the conductive channels in this case should provide maximum electrical conductivity in the direction parallel to the magnetic field lines. In order for the ion-conducting channels in the LB film to be oriented along the layer, the crown ether molecules (their constituents) must be tilted edgewise, which corresponds to the structural models established in the study of films based on crown ethers and a mixture of crown ether and sodium laurate. During the transfer of a monolayer onto a solid substrate, the azimuthal orientation of the conducting channels is preserved, which is confirmed not only by electron diffraction, but also by direct measurements of the planar conductivity of LB films in different directions (Fig. 17). Similar results were also obtained for LB films based on the ternary system disubstituted DB24crown8 – C60 fullerene – terbium complex. Rice. Fig. 17. Electrode configuration and electrical conductivity (G) of an LB film of a mixture of crown ether – sodium laurate – terbium complex with different molar ratios of the components along (direction A) and across (direction B) the magnetic field. Go is the conductivity of the clean substrate. The anisotropy of the planar conductivity of the film increases with a decrease in the concentration of molecules of the terbium complex in the mixture, Fig. . 17. This happens due to a decrease in the perturbing effect of these molecules on the structure of the conducting channels. At the same time, the giant magnetic moments Mo of the molecules of the terbium complex, even in the case of their relatively low concentration, make it possible to orient the domain structure formed by the molecules of the crown ether–sodium laurate or crown ether–C60 complexes. Main results and conclusions 1. It has been shown that in structures with polar symmetry formed by mesogenic acrylates, compensation of dipole moments can occur not only at the level of individual molecules, but also during the formation of dimers from polar molecules. The presence of a chiral fragment sterically prevents the compensation of bond dipole moments both in the molecule and in the molecular packing. The addition of a C = O group to the tail part of the molecule changes the nature of the molecular packing; due to the dipole-dipole interaction, the structure becomes more conservative with respect to the azimuthal mismatch (which explains the formation of the polar Cr-H* phase) and phase separation (in LC mixtures of chiral and achiral acrylates ). An increase in the length of the achiral component in mixtures leads to the formation of a normal smectic with overlapping molecules in adjacent layers. A large azimuthal mismatch is a significant factor preventing the formation of polar layers in these phases. 2. It has been established that homopolymers and copolymers based on chiral and achiral acrylates and their mixtures form smectic structures with polar bilayers. The distribution of chiral and achiral components in the bilayer layers depends on their concentration ratios in the copolymer. In the case of different lengths of the chiral and achiral components in the copolymer and their unequal ratio in the layers of the bilayer, characteristic structural changes are observed inside the same type of smectic phases (a case of a kind of microphase separation). The step of the helicoidal structure increases upon going from the same to the unequal ratio of the chiral and achiral components in the layers of the bilayer. At a low concentration of the chiral component, a chevron structure is observed (for CPL1-325). The way in which the copolymers are oriented has a noticeable effect on their structure. When oriented by a constant electric field up to 1106 V/m, the helicoidal structure remains unspun, the degree of orientation of the layered structure is higher compared to the orientation in a magnetic field. In the case of magnetic orientation, the degree of orientation of the side groups of the copolymer and their translational ordering turn out to be higher. 3. It is shown that at the same ratio of chiral and achiral components in the copolymer, the energy difference between the polar and nonpolar states is minimal, which can facilitate the polarization of the sample in an electric field (which should be significantly greater than 1106 V/m). 4. It has been shown that the reason for the X-ray amorphous structure of the LB film formed from comb-shaped polymer molecules is the limited flexibility of the main chain, which leads to the formation of a loose and uneven floating layer on the water surface. Using spacer monolayers formed, for example, on the basis of lead stearate, it is possible to distinguish between individual layers in the LB film and radiographically see a regular multilayer structure. 5. It has been established that parasubstituted biphenyls form monolayers that are denser and more resistant to collapse than phenyl benzoates. An increase in the concentration of the biphenyl component in the floating monolayers of mixtures also increases their stability. The structure of the tail fragment of molecules most strongly affects the density and stability of monolayers: the presence of a carbonyl group in the tail and an increase in its length leads to an increase in the density and stability of monolayers and biphenyls and phenylbenzoates. 6. It has been shown that regular polar films can be formed from mesogenic para-substituted biphenyls and their mixtures with phenyl benzoates using the LB technology. In this case, the presence of a certain correlation in the structure of the LB films and the structure of the bulk phases of the compounds under study is revealed. Stabilization of the quasi-two-dimensional structure of LB films by UV polymerization is possible only in the absence of screening of C=C bonds by terminal fragments of molecules. 7. It has been established that UV polymerization of homo- and heteromolecular floating monolayers, as a rule, is accompanied by their shrinkage and leads to an increase in stability. However, in the case of a large inclination of the molecules in the monolayer, the side groups of the polymer formed after UV irradiation lie on the water surface, and the monolayer begins to collapse almost simultaneously with the beginning of the movement of the compressive barrier. Theoretical physics ABSTRACT of the dissertation for the degree of Candidate of Physical and Mathematical Sciences Novosibirsk - 2011 «Atkarskaya Agata Sergeevna Isomorphisms of linear groups over associative rings Specialty 01.01.06 mathematical logic, algebra and number theory ABSTRACT of the dissertation for the degree of Candidate of Physical and Mathematical Sciences Moscow 2014 named after M.V. Lomonosov“....” «Ponomarev Ivan Viktorovich STRUCTURES FOR IONIZING RADIATION DETECTORS BASED ON EPITAXIAL GALLIUM ARSENIDE specialty 01.04.10 – semiconductor physics ABSTRACT of the dissertation for the degree of Candidate of Physical and Mathematical Sciences Tomsk – 2011 Laboratory of Semiconductor Physics OSP Siberian Institute of Physics and Technology...» «MIRONOV GENNADY IVANOVICH THE THEORY OF TWO-DIMENSIONAL AND NANODIMENSIONAL SYSTEMS WITH STRONG CORRELATIONS IN THE HUBBARD MODEL 01.04.02 – Theoretical Physics Abstract of the dissertation for the degree of Doctor of Physical and Mathematical Sciences Kazan – 2008 2 IN AND. Ulyanov-Lenina Scientific consultant: Doctor of Physical and Mathematical Sciences, Professor Kochelaev Boris Ivanovich Official opponents:...» "ARBUZOV ANDREY ALEKSANDROVICH Theory and methods of analysis of dielectric spectra described by fractional-power expressions with real and complex conjugate exponents Specialty: 01.04.02 - theoretical physics ABSTRACT of the dissertation for the degree of Candidate of Physical and Mathematical Sciences Kazan - 2009 The work was done at the Department of Theoretical physics of the state educational institution of higher professional education Kazansky ... " MUTINA Albina Rishatovna INTERNAL MORNING MAGNETIC FIELD GRADIENTS IN POROSE MEDIA: EXPERIMENTAL RESEARCH Specialty 01.04.07 – Condensed Matter Physics Abstract of dissertation for the degree of Candidate of Physical and Mathematical Sciences Kazan 2007 Work completed at the Department of Molecular Physics...» « dissertations for the degree of Candidate of Physical and Mathematical Sciences Tomsk 2007. The work was done at the Department of Quantum Field Theory of Tomsk State University. Scientific supervisors: Doctor of Physical and Mathematical Sciences, Professor Semn Leonidovich...» "Selivanov Nikita Ivanovich Influence of intermolecular interactions on photoprocesses of substituted acridine, coumarin and Nile red in solutions and thin films in the Laboratory of Photophysics and Photochemistry of Molecules, Tomsk State University Supervisor: Candidate...» «Pleshinsky Ilya Nikolaevich Redefined boundary value problems and conjugation problems for the Helmholtz equation and the system of Maxwell equations 01.01.02 – differential equations Abstract of the thesis for the degree of Candidate of Physical and Mathematical Sciences Kazan – 2007 The work was done in the state educational institution of higher professional education Kazan State University named after . IN AND. Ulyanov-Lenin Doctor of Physical and Mathematical Sciences,...» “Gadirov Ruslan Magomedtakhirovich Experimental and quantum-chemical study of photoprocesses in substituted coumarins 02.00.04 – physical chemistry Abstract of the dissertation for the degree of candidate of chemical sciences Tomsk – 2007 State Educational Institution of Higher Professional Education Tomsk State University...» “KRUTIKOVA Alla Aleksandrovna SPECTRAL ANALYSIS OF COMPOSITE MATERIALS BASED ON NANOCRYSTALLINE SILICON Specialty: 02.00.02 – Analytical chemistry ABSTRACT of the dissertation for the degree of candidate of chemical sciences Moscow–2007 M.V. Lomonosov Scientific adviser: Doctor of Chemistry, Professor Ishchenko Anatoly Alexandrovich Official...» «Lopukhova Svetlana Vladimirovna ASYMPTOTIC AND NUMERICAL METHODS FOR STUDYING SPECIAL FLOWS OF HOMOGENEOUS EVENTS 05.13.18 Mathematical modeling, numerical methods and software packages Abstract of the dissertation for the degree of Candidate of Physical and Mathematical Sciences Tomsk - 2008 The work was done at the Department of Probability Theory and Mathematical Statistics of the Faculty of Applied Mathematics and Cybernetics GOU VPO Tomsk State University Scientific...» “Wang Qingsheng DEVELOPMENT OF NANOSTRUCTURED CATHODE MATERIAL BASED ON Li2FeSiO4 FOR LITHIUM-ION BATTERIES Specialty 05.16.01 – Metal Science and Thermal Treatment of Metals and Alloys ABSTRACT of the dissertation for the degree of candidate of technical sciences St. Petersburg – 2014 vocational education St. Petersburg State Polytechnic ... " “LUNEV IVAN VLADIMIROVICH STUDY OF THE STRUCTURE AND DIPOLE MOBILITY OF HYDROGEN-BOUND SOLUTIONS BY THE METHOD OF TIME DIELECTRIC SPECTROSCOPY Specialty 01.04.03 – radiophysics ABSTRACT of the dissertation for the degree of Candidate of Physical and Mathematical Sciences Kazan – 2007 The work was done at the Department of Kazan State University of Radio Electronics. Candidate of Physical and Mathematical Sciences, Supervisor: Associate Professor Yu.A. Gusev; candidate..." "KHAZIRISHI ENVER OSMANOVICH QUADRATIVE FORMULA FOR SINGULAR INTEGRALS AND DIRECT METHODS FOR SOLVING SINGULAR INTEGRAL EQUATIONS Specialty 01.01.01 - Mathematical Analysis Abstract of the dissertation for the degree of Candidate of Physical and Mathematical Sciences Kazan - 2009 The work was done at the Department of State University of Mathematical Analysis Ady of Physical and Mathematical Sciences, Professor Gabdulkhaev Bilsur Gabdulkhaevich ... " "Shompolova Olga Igorevna Optimal control of linear systems with irregular mixed constraints and determination of the geometry of the optimal trajectory Specialty 05.13.01 - System analysis, control and information processing (industry) ABSTRACT of the dissertation for the degree of candidate of physical and mathematical sciences Moscow - 2012 STATE BUDGETARY INSTITUTION OF SCIENCE COMPUTING CENTER IM. A.A. DORODNITSYNA RUSSIAN ... " «UDK 517.917 BYKOVA TATYANA SERGEEVNA LYAPUNOVSKAYA REDUCIBILITY OF A LINEAR SYSTEM WITH EFFECT 01.01.02 differential equations ABSTRACT of the dissertation for the degree of Candidate of Physical and Mathematical Sciences Izhevsk – 2005 The work was done in GOU VPO Izhevsk State Technical University. Supervisor: Doctor of Physical and Mathematical Sciences, Professor Tonkov Evgeniy Leonidovich Official opponents: Doctor of Physical and Mathematical Sciences, Professor...» «Garnaeva Guzel Ildarovna OPTICAL TRANSITION EFFECTS IN IMPURITY CRYSTALS IN THE PRESENCE OF EXTERNAL INHOMOGENEOUS ELECTROMAGNETIC FIELDS Specialty 01.04.05 - optics ABSTRACT of the dissertation for the degree of Candidate of Physical and Mathematical Sciences Kazan - 2009 - 2 The work was done at the Department of Physical Educational Faculty of General and Experimental Physics institutions of higher professional education Tatar State...» «Kutuzov Alexander Sergeevich MAGNETIC PROPERTIES AND SPIN KINETICS OF KONDO-LATTICES AND SUPERCONDUCTING CUPRATES WITH YTERBIUM IONS 01.04.02 – Theoretical physics Abstract of the dissertation for the degree of Candidate of Physical and Mathematical Sciences Kazan – 2009 The work was done at the Department of Theoretical Physics of Kazan State University. IN AND. Ulyanov-Lenin. Supervisor: Doctor of Physical and Mathematical Sciences, Professor Kochelaev Boris Ivanovich Official...» The method of forming mono- and multimolecular films was developed by Irving Langmuir and his student Katharina Blodgett in the 1930s. Currently, this technology, called the Langmuir-Blodgett method, is actively used in the production of modern electronic devices. The main idea of the method is the formation of a monomolecular layer of an amphiphilic substance on the water surface and its subsequent transfer to a solid substrate. In the aqueous phase, the molecules of the amphiphilic substance are located on the air-water interface. To form a surface monomolecular layer, the surface layer is compressed using special pistons (see Fig. 1). With successive isothermal compression, the structure of a monomolecular film changes, which passes through a series of two-dimensional states, conventionally referred to as the states of gas, liquid crystal, and solid crystal (see Fig. 2). Thus, knowing the phase diagram of a film, one can control its structure and the physicochemical properties associated with it. The transfer of the film to a solid carrier is carried out by immersion in a solution and subsequent removal of a flat substrate from it, on which a surface film occurs. The process of transferring a monomolecular film can be repeated many times, thus obtaining different multimolecular layers. 
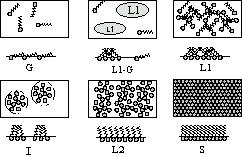
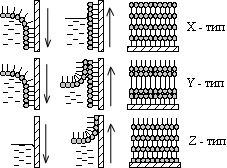
The history of the discovery of Langmuir film
Types of Langmuir films



![]()
Structure of Mesogens in Bulk Samples and Langmuir-Blodgett Films
ALEKSANDROV ANATOLY IVANOVICH
STRUCTURE OF MESOGENS IN VOLUME SAMPLES
AND THE FILMS OF LANGMUIR-BLODGETT
otherwise Langmuir–Blodgett films; Langmuir–Blodgett method(English) abbr., LB) — technology for obtaining mono- and multimolecular films by transferring onto solid Langmuir films (monolayers of compounds formed on the surface of a liquid).
Description
Illustrations
The authors
A source



